Hair cell force generation does not amplify or tune vibrations within the chicken basilar papilla
- PMID: 27796310
- PMCID: PMC5095595
- DOI: 10.1038/ncomms13133
Hair cell force generation does not amplify or tune vibrations within the chicken basilar papilla
Abstract
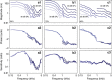
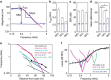
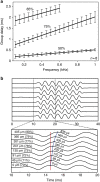
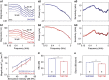
Frequency tuning within the auditory papilla of most non-mammalian species is electrical, deriving from ion-channel resonance within their sensory hair cells. In contrast, tuning within the mammalian cochlea is mechanical, stemming from active mechanisms within outer hair cells that amplify the basilar membrane travelling wave. Interestingly, hair cells in the avian basilar papilla demonstrate both electrical resonance and force-generation, making it unclear which mechanism creates sharp frequency tuning. Here, we measured sound-induced vibrations within the apical half of the chicken basilar papilla in vivo and found broadly-tuned travelling waves that were not amplified. However, distortion products were found in live but not dead chickens. These findings support the idea that avian hair cells do produce force, but that their effects on vibration are small and do not sharpen tuning. Therefore, frequency tuning within the apical avian basilar papilla is not mechanical, and likely derives from hair cell electrical resonance.
Figures



Similar articles
-
Two-Dimensional Cochlear Micromechanics Measured In Vivo Demonstrate Radial Tuning within the Mouse Organ of Corti.J Neurosci. 2016 Aug 3;36(31):8160-73. doi: 10.1523/JNEUROSCI.1157-16.2016. J Neurosci. 2016. PMID: 27488636 Free PMC article.
-
Minimal basilar membrane motion in low-frequency hearing.Proc Natl Acad Sci U S A. 2016 Jul 26;113(30):E4304-10. doi: 10.1073/pnas.1606317113. Epub 2016 Jul 12. Proc Natl Acad Sci U S A. 2016. PMID: 27407145 Free PMC article.
-
Vibration of the organ of Corti within the cochlear apex in mice.J Neurophysiol. 2014 Sep 1;112(5):1192-204. doi: 10.1152/jn.00306.2014. Epub 2014 Jun 11. J Neurophysiol. 2014. PMID: 24920025 Free PMC article.
-
Frequency tuning of mechanical responses in the mammalian cochlea.Biol Res. 1996;29(3):325-31. Biol Res. 1996. PMID: 9278704 Review.
-
The physics of hearing: fluid mechanics and the active process of the inner ear.Rep Prog Phys. 2014 Jul;77(7):076601. doi: 10.1088/0034-4885/77/7/076601. Epub 2014 Jul 9. Rep Prog Phys. 2014. PMID: 25006839 Review.
Cited by
-
Spectrin βV adaptive mutations and changes in subcellular location correlate with emergence of hair cell electromotility in mammalians.Proc Natl Acad Sci U S A. 2017 Feb 21;114(8):2054-2059. doi: 10.1073/pnas.1618778114. Epub 2017 Feb 8. Proc Natl Acad Sci U S A. 2017. PMID: 28179572 Free PMC article.
-
Noise and sensitivity in optical coherence tomography based vibrometry.Opt Express. 2019 Nov 11;27(23):33333-33350. doi: 10.1364/OE.27.033333. Opt Express. 2019. PMID: 31878404 Free PMC article.
-
On the Tonotopy of the Low-Frequency Region of the Cochlea.J Neurosci. 2023 Jul 12;43(28):5172-5179. doi: 10.1523/JNEUROSCI.0249-23.2023. Epub 2023 May 24. J Neurosci. 2023. PMID: 37225436 Free PMC article.
-
Auditory performance in bald eagles and red-tailed hawks: a comparative study of hearing in diurnal raptors.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2019 Dec;205(6):793-811. doi: 10.1007/s00359-019-01367-9. Epub 2019 Sep 13. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2019. PMID: 31520117
-
Interplay between traveling wave propagation and amplification at the apex of the mouse cochlea.Biophys J. 2022 Aug 2;121(15):2940-2951. doi: 10.1016/j.bpj.2022.06.029. Epub 2022 Jun 30. Biophys J. 2022. PMID: 35778839 Free PMC article.
References
-
- Howard J. & Hudspeth A. J. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog's saccular hair cell. Neuron 1, 189–199 (1988). - PubMed
-
- Hudspeth A. J. Integrating the active process of hair cells with cochlear function. Nat. Rev. Neurosci. 15, 600–614 (2014). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

