Endovascular treatment of carotico-cavernous fistulas with acrylic glue: a series of nine cases
- PMID: 27796449
- PMCID: PMC5153414
- DOI: 10.1007/s00234-016-1760-4
Endovascular treatment of carotico-cavernous fistulas with acrylic glue: a series of nine cases
Abstract
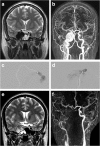
Introduction: Injuries to the internal carotid artery close to the cavernous sinus may result in a fistulous connection between the artery and the venous sinus. Symptoms include pulsatile tinnitus, intracranial bruit, ophthalmological symptoms, and risk of intracerebral hematoma in cases of cortical venous reflux. Previous treatment strategies have included detachable latex balloons, coils, covered stents, or combinations thereof. Today, detachable latex balloons are phased out or withdrawn from several markets. Acrylic glue is a proven stable material used for embolization of arteriovenous shunts. It is a precise, fast, and cost-effective method of endovascular embolization, and it does not cause artifacts on MRI or MRA.
Methods: We treated nine patients suffering from direct fistulas with acrylic glue without any permanent neurological adverse events.
Results: Four patients were treated with glue embolization of the fistula without occlusion of the parent artery. Five patients with long-lasting symptomatology, large tears in the ICA, and with full collateral cerebral circulation were treated with glue embolization of the fistula and sacrifice of the ICA antero- and retrograde via the ICA and the posterior communicating artery.
Conclusion: We suggest acrylic glue to be added to the panel of embolic materials used to treat CCFs.
Keywords: Carotico-cavernous fistula; Cavernous sinus; Collagen disease; Ehlers-Danlos; NBCA.
Conflict of interest statement
Compliance with ethical standardsWe declare that all human and animal studies have been approved by the President of the Group of Reflection on Ethics of Hôpital Foch and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.Conflict of interestWe declare that we have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

