Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging
- PMID: 27796537
- PMCID: PMC5209400
- DOI: 10.1007/s00401-016-1636-z
Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging
Abstract
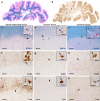
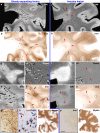
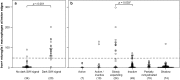
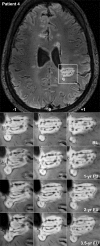
In multiple sclerosis (MS), iron accumulates inside activated microglia/macrophages at edges of some chronic demyelinated lesions, forming rims. In susceptibility-based magnetic resonance imaging at 7 T, iron-laden microglia/macrophages induce a rim of decreased signal at lesion edges and have been associated with slowly expanding lesions. We aimed to determine (1) what lesion types and stages are associated with iron accumulation at their edges, (2) what cells at the lesion edges accumulate iron and what is their activation status, (3) how reliably can iron accumulation at the lesion edge be detected by 7 T magnetic resonance imaging (MRI), and (4) if lesions with rims enlarge over time in vivo, when compared to lesions without rims. Double-hemispheric brain sections of 28 MS cases were stained for iron, myelin, and microglia/macrophages. Prior to histology, 4 of these 28 cases were imaged at 7 T using post-mortem susceptibility-weighted imaging. In vivo, seven MS patients underwent annual neurological examinations and 7 T MRI for 3.5 years, using a fluid attenuated inversion recovery/susceptibility-weighted imaging fusion sequence. Pathologically, we found iron rims around slowly expanding and some inactive lesions but hardly around remyelinated shadow plaques. Iron in rims was mainly present in microglia/macrophages with a pro-inflammatory activation status, but only very rarely in astrocytes. Histological validation of post-mortem susceptibility-weighted imaging revealed a quantitative threshold of iron-laden microglia when a rim was visible. Slowly expanding lesions significantly exceeded this threshold, when compared with inactive lesions (p = 0.003). We show for the first time that rim lesions significantly expanded in vivo after 3.5 years, compared to lesions without rims (p = 0.003). Thus, slow expansion of MS lesions with rims, which reflects chronic lesion activity, may, in the future, become an MRI marker for disease activity in MS.
Keywords: 7 T MRI; Iron rim; Multiple sclerosis; Phase; SWI.
Conflict of interest statement
Compliance with ethical standards The institutional review board of the Medical University in Vienna approved the post-mortem study (EK number 535/2004). No institutional review board was necessary at Vanderbilt University for the post-mortem study. The in vivo study was conducted in Vienna upon local institutional review board approval (EK number: 154/2009). Written informed consent in accordance with the 1964 declaration of Helsinki and its later amendments was obtained from all individual participants included in the study. Conflict of interest The authors declare no conflict of interest with respect to the study and data presented in this paper. Funding This work was supported by funds of the Oesterreichische Nationalbank (Anniversary Fund, project number 16153 to GG and SH and 15680 to ST) and the Austrian Science Fund (FWF project P27744-B27).
Figures



References
-
- Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schroder R, Deckert M, Schmidt S, et al. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

