Cardiac inotropy, lusitropy, and Ca2+ handling with major metabolic substrates in rat heart
- PMID: 27796576
- PMCID: PMC5138277
- DOI: 10.1007/s00424-016-1892-8
Cardiac inotropy, lusitropy, and Ca2+ handling with major metabolic substrates in rat heart
Abstract
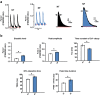
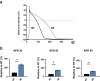
Fatty acid (FA)-dependent oxidation is the predominant process for energy supply in normal heart. Impaired FA metabolism and metabolic insufficiency underlie the failing of the myocardium. So far, FA metabolism in normal cardiac physiology and heart failure remains undetermined. Here, we evaluate the mechanisms of FA and major metabolic substrates (termed NF) on the contraction, relaxation, and Ca2+ handling in rat left ventricular (LV) myocytes. Our results showed that NF significantly increased myocyte contraction and facilitated relaxation. Moreover, NF increased the amplitudes of diastolic and systolic Ca2+ transients ([Ca2+]i), abbreviated time constant of [Ca2+]i decay (tau), and prolonged the peak duration of [Ca2+]i. Whole-cell patch-clamp experiments revealed that NF increased Ca2+ influx via L-type Ca2+ channels (LTCC, ICa-integral) and prolonged the action potential duration (APD). Further analysis revealed that NF shifted the relaxation phase of sarcomere lengthening vs. [Ca2+]i trajectory to the right and increased [Ca2+]i for 50 % of sarcomere relengthening (EC50), suggesting myofilament Ca2+ desensitization. Butanedione monoxime (BDM), a myosin ATPase inhibitor that reduces myofilament Ca2+ sensitivity, abolished the NF-induced enhancement of [Ca2+]i amplitude and the tau of [Ca2+]i decay, indicating the association of myofilament Ca2+ desensitization with the changes in [Ca2+]i profile in NF. NF reduced intracellular pH ([pHi]). Increasing [pH]i buffer capacity with HCO3/CO2 attenuated Δ [pH]i and reversed myofilament Ca2+ desensitization and Ca2+ handling in NF. Collectively, greater Ca2+ influx through LTCCs and myofilament Ca2+ desensitization, via reducing [pH]i, are likely responsible for the positive inotropic and lusitropic effects of NF. Computer simulation recapitulated the effects of NF.
Keywords: Cardiac myocyte; Contraction; Intracellular Ca2+ transient; Metabolic substrates; Myofilament Ca2+ sensitivity; Relaxation; pH.
Conflict of interest statement
Compliance with ethical standards Conflicts of interests The authors declare that they have no conflicts of interest. Funding This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2013068067); by the Brain Korea 21 Graduate Program of the Korean Ministry of Education, Science, and Technology, Seoul National University Hospital; the Korean Society of Hypertension (2013); SK Telecom Research Fund (no. 3420130290); and from the National Natural Science Foundation of China (NSFC, 31460265).
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

