Pentasaccharides for the prevention of venous thromboembolism
- PMID: 27797404
- PMCID: PMC6463830
- DOI: 10.1002/14651858.CD005134.pub3
Pentasaccharides for the prevention of venous thromboembolism
Abstract
Background: Venous thromboembolism (VTE) is a common condition with potentially serious and life-threatening consequences. The standard method of thromboprophylaxis uses an anticoagulant such as low molecular weight heparin (LMWH) or warfarin. In recent years, another type of anticoagulant, pentasaccharide, an indirect factor Xa inhibitor, has shown good anticoagulative effect in clinical trials. Three types of pentasaccharides are available: short-acting fondaparinux, long-acting idraparinux and idrabiotaparinux. Pentasaccharides cause little heparin-induced thrombocytopenia and are better tolerated than unfractionated heparin, LMWH and warfarin. However, no consensus has been reached on whether pentasaccharides are superior or inferior to other anticoagulative methods.
Objectives: To assess effects of pentasaccharides versus other methods of thromboembolic prevention (thromboprophylaxis) in people who require anticoagulant treatment to prevent venous thromboembolism.
Search methods: The Cochrane Vascular Information Specialist (CIS) searched the Specialised Register (last searched March 2016) and the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 2). The CIS searched trial databases for details of ongoing and unpublished studies. Review authors searched LILACS (Latin American and Caribbean Health Sciences) and the reference lists of relevant studies and reviews identified by electronic searches.
Selection criteria: We included randomised controlled trials on any type of pentasaccharide versus other anticoagulation methods (pharmaceutical or mechanical) for VTE prevention.
Data collection and analysis: Two review authors independently selected trials, assessed methodological quality and extracted data in predesigned tables.
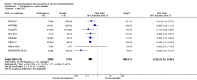
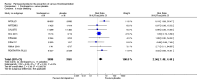
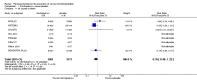
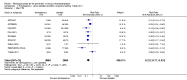
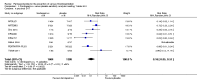
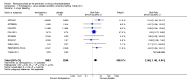
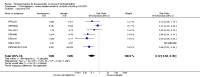
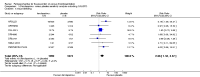
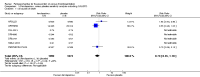
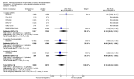
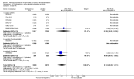
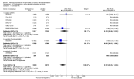
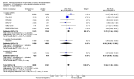
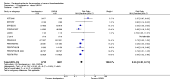
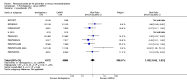
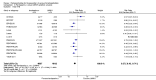
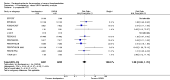
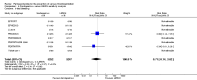
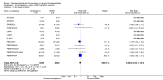
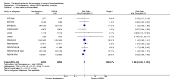
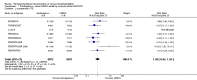
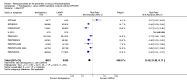
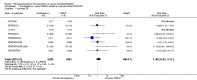
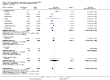
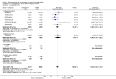
Main results: We included in this review 25 studies with a total of 21,004 participants. All investigated fondaparinux for VTE prevention; none investigated idraparinux or idrabiotaparinux. Studies included participants undergoing abdominal surgery, thoracic surgery, bariatric surgery or coronary bypass surgery; acutely ill hospitalised medical patients; people requiring rigid or semirigid immobilisation; and those with superficial venous thrombosis. Most studies focused on orthopaedic patients. We lowered the quality of the evidence because of heterogeneity between studies and a small number of events causing imprecision.When comparing fondaparinux with placebo, we found less total VTE (risk ratio (RR) 0.24, 95% confidence interval (CI) 0.15 to 0.38; 5717 participants; 8 studies; I2 = 64%; P < 0.00001), less symptomatic VTE (RR 0.15, 95% CI 0.06 to 0.36; 6503 participants; 8 studies; I2 = 0%; P < 0.0001), less total DVT (RR 0.25, 95% CI 0.15 to 0.40; 5715 participants; 8 studies; I2 = 67%; P < 0.00001), less proximal DVT (RR 0.12, 95% CI 0.04 to 0.39; 2746 participants; 7 studies; I2 = 64%; P = 0.0004) and less total pulmonary embolism (PE) (RR 0.16, 95% CI 0.04 to 0.62; 6412 participants; 8 studies; I2 = 0%; P = 0.008) in the fondaparinux group. The quality of the evidence was moderate for total VTE, total DVT and proximal DVT, and high for symptomatic VTE and total PE.When fondaparinux was compared with LMWH, analyses indicated that fondaparinux reduced total VTE and DVT (RR 0.55, 95% CI 0.42 to 0.73; 9339 participants; 11 studies; I2 = 64%; P < 0.0001; and RR 0.54, 95% CI 0.40 to 0.71; 9356 participants; 10 studies; I2 = 67%; P < 0.0001, respectively), and showed a trend toward reduced proximal DVT (RR 0.58, 95% CI 0.33 to 1.02; 8361 participants; 9 studies; I2 = 53%; P = 0.06). Symptomatic VTE (RR 1.03, 95% CI 0.65 to 1.63; 12240 participants; 9 studies; I2 = 35%; P = 0.90) and total PE (RR 1.24, 95% CI 0.65 to 2.34; 12350 participants; 10 studies; I2 = 0%; P = 0.51) indicated no difference between fondaparinux and LMWH. The quality of the evidence was moderate for total VTE, symptomatic VTE, total DVT and total PE, and low for proximal DVT.We showed that fondaparinux increased major bleeding compared with both placebo and LWMH (RR 2.56, 95% CI 1.48 to 4.44; 6659 participants; 8 studies; I2 = 0%; P = 0.0008; moderate-quality evidence; and RR 1.38, 95% CI 1.09 to 1.75; 12,501 participants; 11 studies; I2 = 24%; P = 0.008; high-quality evidence, respectively). All-cause mortality was not different between fondaparinux and placebo or LMWH (RR 0.76, 95% CI 0.48 to 1.22; 6674 participants; 8 studies; I2 = 14%; P = 0.26; moderate-quality evidence; and RR 0.88, 95% CI 0.63 to 1.22; 12,400 participants; 11 studies; I2 = 0%; P = 0.44; moderate-quality evidence, respectively).One study compared fondaparinux with variable and fixed (1 mg per day) doses of warfarin after elective hip or knee replacement surgery and showed no difference in primary and secondary outcomes between fondaparinux and both variable and fixed doses of warfarin. The quality of the evidence was very low. One small study compared fondaparinux with edoxaban in patients with severe renal impairment undergoing lower-limb orthopaedic surgery and reported no thromboembolic events, major bleeding events or deaths in either group. The quality of the evidence was very low. One small study compared fondaparinux with mechanical thromboprophylaxis. Results showed no difference in total VTE and total DVT between fondaparinux and mechanical thromboprophylaxis. This study reported no cases pertaining to the other outcomes of this review. The quality of the evidence was low.There were insufficient studies to permit meaningful conclusions for subgroups of clinical conditions other than orthopaedic surgery.
Authors' conclusions: Moderate to high quality evidence shows that fondaparinux is effective for short-term prevention of VTE when compared with placebo. It can reduce total VTE, DVT, total PE and symptomatic VTE, and does not demonstrate a reduction in deaths compared with placebo. Low to moderate quality evidence shows that fondaparinux is more effective for short-term VTE prevention when compared with LMWH. It can reduce total VTE and total DVT and does not demonstrate a reduction in deaths when compared with LMWH. However, at the same time, moderate to high quality evidence shows that fondaparinux increases major bleeding when compared with placebo and LMWH. Therefore, when fondaparinux is chosen for the prevention of VTE, attention should be paid to the person's bleeding and thrombosis risks. Most data were derived from patients undergoing orthopaedic surgery. Therefore, the conclusion predominantly pertains to these patients. Data on fondaparinux for other clinical conditions are sparse.
Conflict of interest statement
KD: none known. YS: none known. XL: none known. JD: none known. ZG: none known. DL:none known. YZ: none known.
Figures

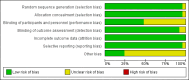













































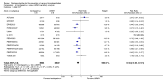

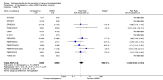
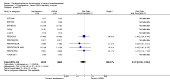

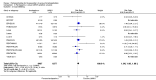











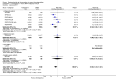
















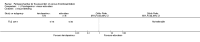
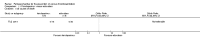

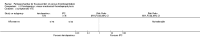


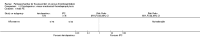
Update of
References
References to studies included in this review
ACT2545 {published data only}
-
- Anon. A multicentre dose finding study of once daily injection of natural pentasaccharide for the prevention of deep vein thrombosis after total hip replacement. GlaxoSmithKline Clinical Trial Register 2008. [URL : www.gsk‐clinicalstudyregister.com/study/ACT2545#rs (accessed 22 July 2014)]
-
- Turpie AG. Pentasaccharide (org31540/sr90107a) clinical trials update: lessons for practice. American Heart Journal 2001;142(2 Suppl):S9‐15. - PubMed
APOLLO {published data only}
-
- Anon. Safety study of fondaparinux sodium to prevent venous thromboembolic events (APOLLO). A multicenter, randomized, double‐blind, parallel group trial to demonstrate the efficacy of fondaparinux sodium in association with intermittent pneumatic compression versus intermittent pneumatic compression used alone for the prevention of venous thromboembolic events in subjects at increased risk undergoing major abdominal surgery. GlaxoSmithKline Clinical Trial Register 2011; Vol. Study No: AR3103414. [URL: www.gsk‐clinicalstudyregister.com/study/103414 (accessed 22 July 2014)]
-
- Priore G, Herzog TJ, Bauer KA, Caprini JA, Comp P, Gent M, et al. Fondaparinux combined with intermittent pneumatic compression (IPC) versus IPC alone in the prevention of venous thromboembolism after major abdominal surgery: results of the APOLLO study. Gynecologic Oncology 2006; Vol. 101:S21.
-
- Turpie AG, Bauer K, Caprini J, Comp P, Gent M, Muntz J. Fondaparinux combined with intermittent pneumatic compression (IPC) versus IPC alone in the prevention of VTE after major abdominal surgery: results of the APOLLO study. Journal of Thrombosis and Haemostasis 2005;3(Suppl 1):Abstract number: P1046. - PubMed
-
- Turpie AG, Bauer KA, Caprini JA, Comp PC, Gent M, Muntz JE, et al. Fondaparinux combined with intermittent pneumatic compression vs. intermittent pneumatic compression alone for prevention of venous thromboembolism after abdominal surgery: a randomized, double‐blind comparison. Journal of Thrombosis and Haemostasis 2007;5(9):1854‐61. - PubMed
-
- Turpie AGG, Bauer KA, Caprini JA, Comp PP, Gent M, Muntz JE. Fondaparinux combined with intermittent pneumatic compression (IPC) versus IPC alone in the prevention of venous thromboembolism after major abdominal surgery: the randomized APOLLO study. Blood 2005;106(11):Abstract 279.
AR3106116 {published data only}
-
- Anon. Clinical evaluation of GSK576428 (fondaparinux sodium) in prevention of venous thromboembolism (VTE) after abdominal surgery. GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/AR3106116 (accessed 22 July 2014)]
-
- Sakon M, Tsukamoto T, Kobayashi T, Fujita S, Kawashima T, Morito M. Clinical evaluation of fondaparinux for prevention of venous thromboembolism after abdominal surgery. A randomized open‐label study of fondaparinux and intermittent pneumatic compression as a benchmark. Rinsho Iyaku ‐ Journal of Clinical Therapeutics & Medicines 2008;24(7):679‐89.
ARTEMIS {published data only}
-
- Anon. A multinational, randomized, double‐blind comparison of once daily subcutaneous fondaparinux sodium with placebo for the prevention of venous thromboembolic events in acutely ill medical patients (ARTEMIS). GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/104619 (accessed 22 July 2014)]
-
- Cohen AT. Thromboprophylaxis with fondaparinux in acutely ill medical patients aged 75 years or more. The Hematology Journal 2004;5(Suppl 2):105.
-
- Cohen AT. Thromboprophylaxis with fondaparinux in acutely ill medical patients with moderate renal impairment. The Hematology Journal 2004;5(Suppl 2):54.
-
- Cohen AT, Davidson BL, Gallus AS. Fondaparinux for the prevention of VTE in acutely ill medical patients. Blood 2003;102(11):15a‐Abstract 42.
Bern 2015 {published data only}
-
- Bern MM, Hazel D, Deeran E, Richmond JR, Ward DM, Spitz DJ, et al. Low dose compared to variable dose warfarin and to fondaparinux as prophylaxis for thromboembolism after elective hip or knee replacement surgery; a randomized, prospective study. Thrombosis Journal 2015;13(32):672‐83. [DOI: 10.1186/s12959-015-0062-0] - DOI - PMC - PubMed
CALISTO {published data only}
-
- Anonymous. Evaluation of fondaparinux (also called ARIXTRA) 2.5 mg subcutaneously once daily for the treatment of superficial thrombophlebitis (also known as superficial vein thrombosis). GlaxoSmithKline Clinical Trial Register 2013. [URL: www.gsk‐clinicalstudyregister.com/study/ART108053 (accessed 22 July 2014)]
-
- Decousus H, Prandoni P, Mismetti P, Bauersachs RM, Boda Z, Brenner B, et al. Fondaparinux for the treatment of superficial‐vein thrombosis in the legs. New England Journal of Medicine 2010;363(13):1222‐32. - PubMed
-
- Leizorovicz A, Becker F, Buchmuller A, Quere I, Prandoni P, Decousus H, et al. Clinical relevance of symptomatic superficial‐vein thrombosis extension: lessons from the CALISTO study. Blood 2013;122:1724‐9. - PubMed
Cho 2013 {published data only}
-
- Cho KY, Kim KI, Khurana S, Bae DK, Jin W. Is routine chemoprophylaxis necessary for prevention of venous thromboembolism following knee arthroplasty in a low incidence population?. Archives of Orthopaedic and Trauma Surgery 2013;133(4):551‐9. - PubMed
DRI4090 {published data only}
-
- Anon. A multicenter, randomized, double‐blind, placebo controlled, parallel group, dose response study of subcutaneous (Org31540/SR90107A) in the prevention of venous thromboembolism after elective total hip replacement surgery. GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/DRI4090 (accessed 22 July 2014)]
DRI4757 {published data only}
-
- Anonymous. A multicenter, randomized, double‐blind, placebo‐controlled, parallel group, dose response study of subcutaneous (Org31540/SR90107A) in the prevention of venous thromboembolism after elective total knee replacement surgery. GlaxoSmithKline Clinical Trial Register 2008; Vol. Study No.: DRI4757. [URL: http://www.gsk‐clinicalstudyregister.com/study/DRI4757?study_ids=DRI4757#rs (accessed 22 July 2014)]
EFFORT {published data only}
-
- Steele KE, Canner J, Prokopowicz G, Verde F, Beselman A, Wyse R, et al. The EFFORT trial. Preoperative enoxaparin versus postoperative fondaparinux for thromboprophylaxis in bariatric surgical patients: a randomized double‐blind pilot trial. Surgery for Obesity and Related Diseases 2015;11(3):672‐83. - PubMed
EPHESUS {published data only}
-
- Anon. European Pentasaccharide Hip Elective Surgery Study (EPHESUS). A multicenter, randomized, double‐blind comparison of once daily subcutaneous (Org31540/SR90107A) with enoxaparin for the prevention of deep vein thrombosis or symptomatic pulmonary embolism in patients undergoing elective hip replacement surgery. GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/63118 (accessed 22 July 2014)]
-
- Borja J, Olivella P, Curto J. Fondaparinux versus enoxaparin for prevention of venous thromboembolism. Lancet 2002;360(9345):1603‐4. - PubMed
-
- Lassen MR. Bauer K, Eriksson BI, Turpie AG, European Pentasaccharide Elective Surgery Study (EPHESUS) Steering Committee. Postoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hip‐replacement surgery: a randomised double‐blind comparison. Lancet 2002;359(9319):1715‐20. - PubMed
FONDACAST {published data only}
-
- Anon. FONDAparinux in patients with a plaster CAST (FONDACAST). GlaxoSmithKline Clinical Trial Register 2011. [URL: www.gsk‐clinicalstudyregister.com/study/109350 (accessed 22 July 2014)]
-
- Samama CM, Lecoules N, Kierzek G, Claessens YE, Riou B, Rosencher N, et al. Comparison of fondaparinux with low molecular weight heparin for venous thromboembolism prevention in patients requiring rigid or semi‐rigid immobilization for isolated non‐surgical below‐knee injury. Journal of Thrombosis and Haemostasis 2013;11(10):1833‐43. - PubMed
-
- Samama CM, Lecoules N, Kierzek G, Claessens YE, Riou B, Rosencher N, et al. Comparison of fondaparinux with low‐molecular‐weight heparin for venous thromboembolism prevention in patients requiring rigid or semi‐rigid immobilization for isolated non‐surgical below‐knee injury. Annales Francaises de Medecine d'Urgence 2014;4(3):153‐66. - PubMed
-
- Samama CM, Riou B, Roy P‐M, Sautet A, Mismetti P, FONDACAST Study Group. Prevention of venous thromboembolism after an isolated, non‐surgical below‐knee injury. Benefit/risk of fondaparinux vs. low molecular weight heparin: the Fondacast study. Journal of Thrombosis and Haemostasis 2013;11(Suppl S3):5. - PubMed
-
- Samama CMR. Subgroup analysis of the FONDACAST study comparing fondaparinux to low‐molecular‐weight heparin for the prevention of venous thromboembolism after an isolated, non‐surgical below‐knee injury. Journal of Thrombosis and Haemostasis 2013;11 (Suppl S2):88. - PubMed
Fuji 2015 {published and unpublished data}
Kolluri 2016 {published data only}
-
- Kolluri R, Plessa AL, Sanders MC, Singh NK, Lucore C. A randomized study of the safety and efficacy of fondaparinux versus placebo in the prevention of venous thromboembolism after coronary artery bypass graft surgery. American Heart Journal 2016;171(1):1‐6. - PubMed
L‐8541 {published data only}
-
- Anon. Arixtra VTE Prevention Study ‐ Fondaparinux compared with enoxaparin in the prevention of venous thromboembolism (VTE) in Chinese patients undergoing elective knee replacement, hip surgery or a revision of components. GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/L‐8541 (accessed 22 July 2014)]
L‐8635 {published data only}
-
- Anon. Fondaparinux compared with enoxaparin in the prevention of venous thromboembolism in Taiwanese patients undergoing elective knee replacement. GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/L‐8635 (accessed 22 July 2014)]
Li 2015 {published and unpublished data}
-
- Li B, Wang K, Zhao X, Lin C, Sun H. Comparison of fondaparinux sodium and low molecular weight heparin in the treatment of hypercoagulability secondary to traumatic infection. Chinese Journal of Traumatology 2015;18:147‐9. - PubMed
PEGASUS {published data only}
-
- Agnelli G, Bergqvist D, Cohen A, Gallus A, Gent M. A randomized double‐blind study to compare the efficacy and safety of fondaparinux with dalteparin in the prevention of venous thromboembolism after high‐risk abdominal surgery: the Pegasus study. Journal of Thrombosis and Haemostasis 2003; Vol. 1, issue Suppl 1:Abstract OC006.
-
- Agnelli G, Bergqvist D, Cohen A, Gallus AS, Gent M. A randomized double‐blind study to compare the efficacy and safety of postoperative fondaparinux (Arixtra) and preoperative dalteparin in the prevention of venous thromboembolism after high‐risk abdominal surgery: the PEGASUS study. Blood 2003;102(11 Pt 1):Abstract 40.
-
- Agnelli G, Bergqvist D, Cohen AT, Gallus AS, Gent M, PEGASUS Investigators. Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high‐risk abdominal surgery. British Journal of Surgery 2005;92(10):1212‐20. - PubMed
-
- Anon. A multicenter, multinational, randomized, double‐blind study to compare the efficacy and safety of fondaparinux sodium (Org31540/SR90107A) with dalteparin (Fragmin) in the prevention of venous thromboembolic events in high‐risk abdominal surgery (PEGASUS). GlaxoSmithKline Clinical Trial Register 2005. [URL: www.gsk‐clinicalstudyregister.com/study/EFC3557 (accessed 22 July 2014)]
PENTAMAKS {published data only}
-
- Anon. A multicenter, multinational, randomized, double‐blind comparison of subcutaneous (Org31540/SR90107A) with enoxaparin in the prevention of deep vein thrombosis and symptomatic pulmonary embolism after elective major knee surgery or a revision (PENTAMAKS). GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/095‐002 (accessed 22 July 2014)]
-
- Bauer K. The PENTAMAKS Study. Comparison of the first synthetic factor Xa inhibitor with low molecular weight heparin in the prevention of venous thromboembolism (VTE) after elective major knee surgery. Blood 2000;96:490a.
-
- Bauer KA, Eriksson BI, Lassen MR, Turpie AGG, for the Steering Committee of the Pentasaccharide in Major Knee Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. New England Journal of Medicine 2001;345(18):1305‐10. - PubMed
PENTATHLON {published data only}
-
- Turpie AG. Pentasaccharide (Org31540/SR90107A) clinical trials update: lessons for practice. American Heart Journal 2001;142(2 Suppl):S9‐15. - PubMed
-
- Turpie AG, Gallus AS, Hoek JA, Pentasaccharide Investigators. A synthetic pentasaccharide for the prevention of deep‐vein thrombosis after total hip replacement. New England Journal of Medicine 2001;344(9):619‐25. - PubMed
PENTATHLON 2000 {published data only}
-
- Anon. A multicenter, multinational, randomized, double‐blind comparison of subcutaneous (Org31540/SR90107A) with enoxaparin in the prevention of deep vein thrombosis and symptomatic pulmonary embolism after elective hip replacement or a revision. PENTATHLON 2000. GlaxoSmithKline Clinical Trial 2008. [URL: www.gsk‐clinicalstudyregister.com/study/EFC2442 (accessed 22 July 2014)]
-
- Turpie AG. The PENTATHALON 2000 Study: comparison of the first synthetic factor Xa inhibitor with low molecular weight heparin in the prevention of venous thromboembolism. Blood 2000;96(491A):Abstract A2112.
-
- Turpie AG, Bauer KA, Eriksson BI, Lassen MR, PENTATHALON 2000 Study Steering Committee. Postoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip‐replacement surgery: a randomised double‐blind trial. Lancet 2002;359(9319):1721‐6. - PubMed
PENTHIFRA {published data only}
-
- Anon. A multicenter, multinational, randomized double‐blind comparison study of subcutaneous (Org31540/SR90107A) versus enoxaparin 40 mg o.d. in the prevention of deep vein thrombosis and symptomatic pulmonary embolism in hip fracture surgery (PENTHIFRA). GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/EFC2698 (accessed 22 July 2014)]
-
- Eriksson B. The PENTHIFRA Study. Comparison of the first synthetic Factor Xa inhibitor with low molecular weight heparin (LMWH) for the prevention of venous thromboembolism (VTE) after hip fracture surgery. Blood 2000;96(490A):Abstract 2110.
-
- Eriksson BI, Bauer KA, Lassen MR, Turpie AGG, for the Steering Committee of the Pentasaccharide in Hip‐Fracture Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip‐fracture surgery. The New England Journal of Medicine 2001;345(18):1298‐304. - PubMed
PENTHIFRA PLUS {published data only}
-
- Anon. A multicenter, multinational, randomized, double‐blind study of fondaparinux sodium (Org31540/SR90107A) versus placebo for the prolonged prevention of VTE in hip fracture surgery (PENTIHFRA PLUS). GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/EFC4582?study_ids=EFC4582#rs (accessed 22 July 2014)]
-
- Bauer KA, Eriksson BI, Lassen MR, Turpie AGG. No episode of thrombocytopenia after four‐week administration of fondaparinux, a new synthetic and selective inhibitor of factor Xa, in the PENTHIFRA‐PLUS study. Journal of Thrombosis and Haemostasis 2003;1(Suppl 1):P2050.
-
- Dobesh PP. Novel concepts: emerging data and the role of extended prophylaxis following hip fracture surgery. American Journal of Health‐System Pharmacy 2003;60(22 Suppl 7):S15‐9. - PubMed
-
- Eriksson BI. A multicenter, randomized, placebo‐controlled, double‐blind study of fondaparinux for the prolonged prevention of venous thromboembolism in hip fracture surgery. Abstracts of the Sicot/Sirot XXII World Congress 2002:318.
-
- Eriksson BI, Lassen MR. Consistency of efficacy of extended thromboprophylaxis with fondaparinux (Arixtra) in prevention of venous thromboembolism (VTE) after hip fracture surgery according to different composite efficacy endpoints: the PENTHIFRA‐PLUS study. Journal of Thrombosis and Haemostasis 2003;1(Suppl 1):Abstract P2064.
Shen 2014 {published and unpublished data}
-
- Shen Y, Zhong M, Tan L. Fondaparinux versus low molecular weight heparin following esophagectomy: results from a randomized and controlled trial. Chest 2014;145(3_Meeting Abstracts):535D.
Yokote 2011 {published data only}
-
- Yokote R, Matsubara M, Hirasawa N, Hagio S, Ishii K, Takata C. Is routine chemical thromboprophylaxis after total hip replacement really necessary in a Japanese population?. Journal of Bone and Joint Surgery British Volume 2011;93(2):251‐6. - PubMed
References to studies excluded from this review
ACT1840 {published data only}
-
- Anon. A multicentre pilot study of natural pentasaccharide (SR90107A /Org31540) for the prevention of deep venous thrombosis after total hip replacement. GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/ACT1840 (accessed 22 July 2014)]
Amadeus 2008 {published data only}
-
- Amadeus Investigators, Bousser MG, Bouthier J, Büller HR, Cohen AT, Crijns H, Davidson BL, et al. Comparison of idraparinux with vitamin K antagonists for prevention of thromboembolism in patients with atrial fibrillation: a randomised, open‐label, non‐inferiority trial. Lancet 2008;371(9609):315‐21. [DOI: 10.1016/S0140-6736(08)60168-3] - DOI - PubMed
AR3106206 {published data only}
-
- Anon. Clinical evaluation of GSK576428 (fondaparinux sodium) in the treatment of acute pulmonary thromboembolism (PE). GlaxoSmithKline Clinical Trial Register 2009. [NCT00981409; URL: www.gsk‐clinicalstudyregister.com/study/106206?study_ids=AR3106206#ps (accessed 22 July 2014)]
AR3106333 {published data only}
-
- Anon. Clinical evaluation of GSK576428 (fondaparinux sodium) in prevention of venous thromboembolism after elective total hip replacement surgery. GlaxoSmithKline Clinical Trial Register 2008. [URL: http://www.gsk‐clinicalstudyregister.com/study/AR3106333?study_ids=AR3106333#ps (accessed 22 July 2014)]
AR3106335 {published data only}
-
- Anon. Clinical evaluation of GSK576428 (fondaparinux sodium) in prevention of venous thromboembolism after hip fracture surgery. GlaxoSmithKline Clinical Trial Register 2008. [NCT00320424; URL: http://www.gsk‐clinicalstudyregister.com/study/AR3106335?study_ids=AR3106335#ps (accessed 22 July 2014)]
Argun 2013 {published data only}
Bonneux 2006 {published data only}
-
- Bonneux IM, Bellemans J, Fabry G. Evaluation of wound healing after total knee arthroplasty in a randomized prospective trial comparing fondaparinux with enoxaparin. Knee 2006;13(2):118‐21. - PubMed
Buller 2014 {published data only}
-
- Buller HR, Halperin J, Hankey GJ, Pillion G, Prins MH, Raskob GE. Comparison of idrabiotaparinux with vitamin K antagonists for prevention of thromboembolism in patients with atrial fibrillation: the Borealis‐Atrial Fibrillation Study. Journal of Thrombosis and Haemostasis 2014;12(6):824‐30. - PubMed
Cassiopea {published data only}
-
- Büller HR, Gallus AS, Pillion G, Prins MH, Raskob GE, on behalf of the Cassiopea Investigators. Enoxaparin followed by once‐weekly idrabiotaparinux versus enoxaparin plus warfarin for patients with acute symptomatic pulmonary embolism: a randomised, double‐blind, double‐dummy, non‐inferiority trial. Lancet 2012;379(9811):123‐9. - PubMed
Cohen 2007 {published data only}
-
- Cohen A, Brenkel I, Skinner J, Warwick D. Graduated compression stockings do not add to the efficacy of fondaparinux for thromboprophylaxis in hip replacement surgery. A multi‐centre, multi‐national, randomised, open‐label study. Journal of Bone and Joint Surgery British Volume 2009;91‐B(Suppl 1):73‐c.
-
- Cohen AT, Skinner JA, Warwick D, Brenkel I. The use of graduated compression stockings in association with fondaparinux in surgery of the hip. A multicentre, multinational, randomised, open‐label, parallel‐group comparative study. Journal of Bone and Joint Surgery British Volume 2007;89(7):887‐92. - PubMed
EQUINOX {published data only}
-
- The EQUINOX Invesstigators. Efficacy and safety of once weekly subcutaneous idrabiotaparinux in the treatment of patients with symptomatic deep venous thrombosis. Journal of Thrombosis and Haemostasis 2011;9(1):92‐9. - PubMed
extended van Gogh {published data only}
-
- van Gogh Investigators, Buller HR, Cohen AT, Davidson B, Decousus H, Gallus AS, et al. Extended prophylaxis of venous thromboembolism with idraparinux. New England Journal of Medicine 2007;357(11):1105‐12. - PubMed
FLEXTRA {published data only}
-
- Anon. Flexibility in administration of Arixtra for prevention of symptomatic venous thromboembolism in orthopedic surgery. GlaxoSmithKline Clinical Trial Register 2008. [URL: http://www.gsk‐clinicalstudyregister.com/study/L8518?study_ids=L851#rs (accessed 22 July 2014)]
-
- Colwell CW Jr, Kwong LM, Turpie AGG, Davidson BL. Flexibility in administration of fondaparinux for prevention of symptomatic venous thromboembolism in orthopaedic surgery. Journal of Arthroplasty 2006;21(1):36‐45. - PubMed
-
- Davidson BL, Turpie AGG, Kwong LM, Colwell CW. FLEXTRA: early vs delayed initiation of postoperative fondaparinux prophylaxis after joint replacement: a clinical outcome study. Journal of Thrombosis and Haemostasis 2005;3(Suppl 1):Abstract OR061.
Kawaji 2012 {published data only}
-
- Kawaji H, Ishii M, Tamaki Y, Hamasaki M, Ishikawa H, Sasaki K, et al. Postoperative prophylactic effect of fondaparinux for prevention of deep venous thrombosis after cemented total hip replacement:a comparative study. Modern Rheumatology / The Japan Rheumatism Association 2012;22(2):216–22. - PubMed
Li 2013 {published data only}
-
- Li MW, Zhao XM, Rao LX. The clinical efficacy and safety of fondaparinux combined with tirofiban hydrochloride in patients with acute coronary syndrome undergoing complex percutaneous coronary intervention. Zhonghua Nei Ke Za Zhi 2013;52:1037‐40. - PubMed
MATISSE‐DVT {published data only}
-
- Buller HR, Davidson BL, Decousus H, Gallus A, Gent M, Piovella F, et al. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Annals of Internal Medicine 2004;140(11):867‐73. - PubMed
MATISSE‐PE {published data only}
-
- The Matisse Investigators. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. New England Journal of Medicine 2003;349(18):1695‐702. - PubMed
NCT00521885 {published data only}
-
- NCT00521885. Comparison of arixtra vs. lovenox to prevent blood clots in medically ill patients (BRiEF). ClinicalTrials.gov 2007. [URL: http://clinicaltrials.gov/show/NCT00521885 (accessed 8 March 2016)]
NCT00539942 {published data only}
-
- NCT00539942. Study of arixtra to prevent blood clots in women undergoing abdominopelvic surgery for likely gynecologic malignancy. ClinicalTrials.gov. [URL: http://clinicaltrials.gov/ct2/show/NCT00539942 (accessed 8 March 2016)]
PENTATAK {published data only}
-
- Anon. A multicenter, concurrent control, randomized, open‐label, assessor‐blind, dose‐ranging study (of Org 31540/SR90107A) in the prophylaxis of deep vein thrombosis in subjects undergoing total knee replacement surgery (PENTATAK). GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/95001 (accessed 23 July 2014)]
PERSIST {published data only}
-
- PERSIST Investigators. A novel long‐acting synthetic factor Xa inhibitor (SanOrg34006) to replace warfarin for secondary prevention in deep vein thrombosis. A phase II evaluation. Journal of Thrombosis and Haemostasis 2004;2(1):47‐53. - PubMed
Rembrandt {published data only}
-
- The Rembrandt Investigators. Treatment of proximal deep vein thrombosis with a novel synthetic compound (SR90107A/ Org31540) with pure anti‐factor Xa activity. A phase II evaluation. Circulation 2000;102:2726‐31. - PubMed
SAFE‐AF {published data only}
-
- Cohen A, Stellbrink C, Heuzey JY, Faber T, Aliot E, Banik N, et al. Safety of fondaparinux in transoesophageal echocardiography‐guided electric cardioversion of atrial fibrillation (SAFE‐AF) study: a pilot study. Archives of Cardiovascular Diseases 2014;108:122‐31. - PubMed
Sasaki 2009 {published data only}
-
- Sasaki S, Miyakoshi N, Matsuura H, Saitoh H, Kudoh D, Shimada Y. Prospective randomized controlled trial on the effect of fondaparinux sodium for prevention of venous thromboembolism after hip fracture surgery. Journal of Orthopaedic Science 2009;14(5):491‐6. - PubMed
Sasaki 2011 {published data only}
-
- Sasaki S, Miyakoshi N, Matsuura H, Saito H, Nakanishi T, Kudo Y, et al. Prospective study on the efficacies of fondaparinux and enoxaparin in preventing venous thromboembolism after hip fracture surgery. Journal of Orthopaedic Science 2011;16(1):64‐70. - PubMed
Savi 2005 {published data only}
-
- Savi P, Chong BH, Greinacher A, Gruel Y, Kelton JG, Warkentin TE, et al. Effect of fondaparinux on platelet activation in the presence of heparin‐dependent antibodies: a blinded comparative multicenter study with unfractionated heparin. Blood 2005;105(1):139‐44. - PubMed
Tsutsumi 2012 {published data only}
-
- Tsutsumi S, Yajima R, Tabe Y, Takaaki T, Fujii F, Morita H, et al. The efficacy of fondaparinux for the prophylaxis of venous thromboembolism after resection for colorectal cancer. Hepatogastroenterology 2012;59:2477‐9. - PubMed
van Gogh‐DVT {published data only}
-
- van Gogh Investigators, Buller HR, Cohen AT, Davidson B, Decousus H, Gallus AS, et al. Idraparinux versus standard therapy for venous thromboembolic disease. New England Journal of Medicine 2007;357(11):1094‐104. - PubMed
van Gogh‐PE {published data only}
-
- van Gogh Investigators. Idraparinux versus standard therapy for venous thromboembolic disease. New England Journal of Medicine 2007;357(11):1094‐104. - PubMed
Xin 2013 {published data only}
-
- Xin Z, Ya‐Ling H, Xiao‐Zeng W, Bai‐Ge X. Efficacy and safety of fondaparinux during thrombolytic therapy in acute ST elevation myocardial infarction patients. Heart 2013;99:A54‐A55.
Yamaoka 2014 {published data only}
-
- Yamaoka YI, Nishikawa T. The impact of fondaparinux on the prophylaxis of venous thromboembolism: the efficacy, safety and prognosis after resection for colorectal cancer. Annals of Surgical Oncology. 2014; Vol. 21, issue 1 Suppl 1.
Zhao 2013 {published data only}
-
- Zhao X, Han Y, Wang X, Xu B. Efficacy and safety of fondaparinux during thrombolytic therapy in acute ST elevation myocardial infarction patients. Cardiology (Switzerland) 2013;126:138.
Zhao 2015 {published data only}
-
- Zhao XM, Gao CY, Chu YJ. Fondaparinux vs. enoxaparin in patients with non‐ST elevation acute coronary syndromes (NSTE‐ACS) treated with percutaneous coronary intervention and tirofiban: an exploratory study in China. Journal of Clinical Pharmacy and Therapeutics 2015;40:584‐9. - PubMed
References to ongoing studies
EUCTR2007‐003746‐15‐DE {published data only}
-
- EUCTR2007‐003746‐15‐DE. Prospective randomized open study on the comparison of fondaparinux with the low‐molecular‐weight heparin enoxaparin in patients undergoing femoro‐distal venous bypass operation. WHO International Clinical Trials Registry 2008. [URL: http://apps.who.int/trialsearch/Trial.aspx?TrialID=EUCTR2007‐003746‐15‐DE (accessed 19 September 2016)]
EUCTR2008‐001779‐31‐IT {published data only}
-
- Markers of hypercoagulability and risk of death and rehospitalization in heart failure patients: a pilot study on the effects of Fondaparinux ‐ Fondaparinux and heart failure. WHO International Clinical Trials Registry. [URL : http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2008‐001779‐31‐IT (accessed 19 September 2016)]
JPRN‐UMIN000002444 {published data only}
-
- JPRN‐UMIN000002444. Randomized study of anti‐coagulant therapy to prevent postoperative deep venous thrombosis/pulmonary embolism. WHO International Clinical Trials Registry 2009. [URL: http://apps.who.int/trialsearch/trial.aspx?trialid=JPRN‐UMIN000002444 (accessed 19 September 2016)]
JPRN‐UMIN000007005 {published data only}
-
- JPRN‐UMIN000007005. The efficacy and safety of anticoagulant therapy Arixtra Injection for the prevention of the vein thromboembolism in laparoscopic colorectal surgery. WHO International Clinical Trials Registry 2011. [URL: http://apps.who.int/trialsearch/Trial.aspx?TrialID=JPRN‐UMIN000007005 (accessed 19 September 2016)]
JPRN‐UMIN000008435 {published data only}
-
- Phase III study of efficacy of fondaparinux on the prevention of post‐operative venous thromboembolism in patients undergoing with laparoscopic colorectal cancer surgery. WHO International Clinical Trials Registry. [URL: http://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000008435 (accessed 19 September 2016)]
PROTECT {published data only}
-
- NCT00881088. Prophylaxis of thromboembolic complications trial: thromboprophylaxis needed in below knee plaster cast immobilization for ankle and foot fractures (PROTECT). ClinicalTrials.gov 2009. [URL: http://clinicaltrials.gov/ct2/show/NCT00881088?term=NCT00881088&rank=1 (accessed 8 March 2016)]
Additional references
Ageno 2012
-
- Ageno W, Riva N, Noris P, Nisio M, Regina M, Arioli D, et al. The FONDAIR Study Group. Safety and efficacy of low dose fondaparinux (1.5 mg) for the prevention of venous thromboembolism in acutely ill medical patients with renal impairment: the FONDAIR study. Journal of Thrombosis and Haemostasis 2012;10(11):2291‐7. - PubMed
Agnelli 2000
-
- Agnelli G, Sonaglia F. Prevention of venous thromboembolism. Thrombosis Research 2000;97(1):V49‐62. - PubMed
Amiral 1997
-
- Amiral J, Lormeau JC, Marfaing‐Koka A, Vissac AM, Wolf M, Boyer‐Neumann C, et al. Absence of cross‐reactivity of SR90107A/ORG31540 pentasaccharide with antibodies to heparin‐PF4 complexes developed in heparin‐induced thrombocytopenia. Blood Coagulation and Fibrinolysis 1997;8(2):114‐7. - PubMed
Atkins 2004
Bauer 2003
-
- Bauer KA. New pentasaccharides for prophylaxis of deep vein thrombosis: pharmacology. Chest 2003;124(6 Suppl):364s‐70s. - PubMed
Bauersachs 2005
-
- Bauersachs RM. Fondaparinux: an update on new study results. European Journal of Clinical Investigation 2005;35(Suppl 1):27‐32. - PubMed
Bergqvist 2006
Bounameaux 2002
-
- Bounameaux H, Perneger T. Fondaparinux: a new synthetic pentasaccharide for thrombosis prevention. Lancet 2002;359(9319):1710‐1. - PubMed
Brito 2011
Delavenne 2010
-
- Delavenne X, Zufferey P, Baylot D, Nguyen P, Borg JY, Fontenay M, et al. Population pharmacokinetics of fondaparinux administered at prophylactic doses after major orthopaedic surgery in everyday practice. Thrombosis and Haemostasis 2010;104(2):252‐60. - PubMed
Delavenne 2012
-
- Delavenne X, Zufferey P, Nguyen P, Rosencher N, Samama CM, Bazzoli C, et al. Pharmacokinetics of fondaparinux 1.5 mg once daily in a real‐world cohort of patients with renal impairment undergoing major orthopaedic surgery. European Journal of Clinical Pharmacology 2012;68(10):1403‐10. - PubMed
Erkens 2010
Falck‐Ytter 2012
-
- Falck‐Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e278S‐325S. - PMC - PubMed
Fowkes 2003
-
- Fowkes FJ, Price JF, Fowkes FG. Incidence of diagnosed deep vein thrombosis in the general population: systematic review. European Journal of Vascular and Endovascular Surgery 2003;25(1):1‐5. - PubMed
Geerts 2001
-
- Geerts WH, Heit JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA, et al. Prevention of venous thromboembolism. Chest 2001;119(1 Suppl):132S‐75S. - PubMed
Geerts 2004
-
- Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126(3 Suppl):338S‐400S. - PubMed
Geerts 2008
-
- Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practive Guidelines (8th Edition). Chest 2008;133(6 Suppl):381S–453S. - PubMed
GradePro [Computer program]
-
- Brozek J, Oxman A, Schünemann H. [Computer program on www.gradepro.org]. GRADEpro. Version December 2014. McMaster University, 2014.
Hansson 2000
-
- Hansson PO, Sorbo J, Eriksson H. Recurrent venous thromboembolism after deep vein thrombosis: incidence and risk factors. Archives of Internal Medicine 2000;160(6):769‐74. - PubMed
Heit 2001
-
- Heit JA, Melton LJ 3rd, Lohse CM, Petterson TM, Silverstein MD, Mohr DN, et al. Incidence of venous thromboembolism in hospitalised patients vs community residents. Mayo Clinic Proceedings 2001;76(11):1102‐10. - PubMed
Heit 2005
-
- Heit JA, Cohen AT, Anderson FA Jr. Estimated annual number of incident and recurrent, non‐fatal and fatal venous thromboembolism (VTE) events in the US. Blood 2005;106(11):Abstract 910.
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011], The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011a
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hills 2009
-
- Hills RK, Gray R, Wheatley K. Balancing treatment allocations by clinician or center in randomized trials allows unacceptable levels of treatment prediction. Journal of Evidence‐based Medicine 2009;2:196–204. - PubMed
Koopman 2003
-
- Koopman MM, Buller HR. Short‐ and long‐acting synthetic pentasaccharides. Journal of Internal Medicine 2003;254(4):335‐42. - PubMed
Metzger 2015
Mismetti 2012
-
- Mismetti P, Samama CM, Rosencher N, Vielpeau C, Nguyen P, Deygas B, et al. Venous thromboembolism prevention with fondaparinux 1.5 mg in renally impaired patients undergoing major orthopaedic surgery. A real‐world, prospective, multicentre, cohort study. Thrombosis and Haemostasis 2012;107(6):1151‐60. - PubMed
Nijkeuter 2004
-
- Nijkeuter M, Huisman MV. Pentasaccharides in the prophylaxis and treatment of venous thromboembolism: a systematic review. Current Opinion in Pulmonary Medicine 2004;10:338‐44. - PubMed
Olson 1992
-
- Olson ST, Bjork I, Sheffer R, Craig PA, Shore JD, Choay J. Role of the antithrombin‐binding pentasaccharide in heparin acceleration of antithrombin‐proteinase reactions. Resolution of the antithrombin conformational change contribution to heparin rate enhancement. Journal of Biological Chemistry 1992;267(18):12528‐38. - PubMed
Palareti 2012
-
- Palareti G, Schellong S. Isolated distal deep vein thrombosis: what we know and what we are doing. Journal of Thrombosis and Haemostasis 2012;10(1):11‐9. - PubMed
Paty 2007
-
- Paty I, Trellu M, Paquet N, Sibille M, Perez Y, Ortiz J. Neutralization by avidin of anticoagulant activity of biotinylated idraparinux, the first and unique reversible long‐acting anticoagulant. Journal of Thrombosis and Haemostasis. 2007; Vol. 5 (Suppl 2):O‐T‐050.
Paty 2010
-
- Paty I, Trellu M, Destors JM, Cortez P, Boelle E, Sanderink G. Reversibility of the anti‐FXa activity of idrabiotaparinux (biotinylated idraparinux) by intravenous avidin infusion. Journal of Thrombosis and Haemostasis 2010;8(4):722‐9. - PubMed
Pinede 2001
-
- Pinede L, Ninet J, Duhaut P, Chabaud S, Demolombe‐Rague S, Durieu I, et al. Comparison of 3 and 6 months of oral anticoagulant therapy after a first episode of proximal deep vein thrombosis or pulmonary embolism and comparison of 6 and 12 weeks of therapy after isolated calf deep vein thrombosis. Circulation 2001;103(20):2453‐60. - PubMed
Prandoni 2008
-
- Prandoni P, Tormene D, Perlati M, Brandolin B, Spiezia L. Idraparinux: review of its clinical efficacy and safety for prevention and treatment of thromboembolic disorders. Expert Opinion on Investigational Drugs 2008;17(5):773‐7. - PubMed
Salazar 2010
-
- Salazar CA, Malaga G, Malasquez G. Direct thrombin inhibitors versus vitamin K antagonists or low molecular weight heparins for prevention of venous thromboembolism following total hip or knee replacement. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD005981.pub2] - DOI - PMC - PubMed
Savi 2008
-
- Savi P, Herault JP, Duchaussoy P, Millet L, Schaeffer P, Petitou M, et al. Reversible biotinylated oligosaccharides: a new approach for a better management of anticoagulant therapy. Journal of Thrombosis and Haemostasis 2008;6(10):1697‐706. - PubMed
Shoda 2015
-
- Shoda N, Yasunaga H, Horiguchi H, Fushimi K, Matsuda S, Kadono Y, et al. Prophylactic effect of fondaparinux and enoxaparin for preventing pulmonary embolism after total hip or knee arthroplasty: a retrospective observational study using the Japanese Diagnosis Procedure Combination database. Modern Rheumatology / The Japan Rheumatism Association 2015;25(4):625‐9. [DOI: 10.3109/14397595.2014.997424] - DOI - PubMed
Southworth 2013
-
- Southworth MR, Reichman ME, Unger EF. Dabigatran and post marketing reports of bleeding. New England Journal of Medicine 2013;368:1272‐4. - PubMed
Stoldt 1997
-
- Stoldt HS, Aftab F, Chinol M, Paganelli G, Luca F, Testori A, et al. Pretargeting strategies for radio‐immuno guided tumour localisation and therapy. European Journal of Cancer 1997;33(2):186‐92. - PubMed
Turpie 2003
-
- Turpie AG, Eriksson BI, Bauer KA, Lassen MR. New pentasaccharides for the prophylaxis of venous thromboembolism: clinical studies. Chest 2003;124(6 Suppl):371S‐8S. - PubMed
Turpie 2003a
-
- Turpie AG. Future therapeutic directions for factor Xa inhibition in the prophylaxis and treatment of thrombotic disorders. American Journal of Health‐System Pharmacy 2003;60(22 Suppl 7):S20‐4. - PubMed
Uchino 2012
-
- Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta‐analysis of non inferiority randomized controlled trials. Archives of Internal Medicine 2012;172:397–402. - PubMed
Veyrat‐Follet 2009
-
- Veyrat‐Follet C, Vivier N, Trellu M, Dubruc C, Sanderink GJ. The pharmacokinetics of idraparinux, a long‐acting indirect factor Xa inhibitor: population pharmacokinetic analysis from Phase III clinical trials. Journal of Thrombosis and Haemostasis 2009;7(4):559‐65. - PubMed
Yusuf 1985
-
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomised trials. Progress in Cardiovascular Diseases 1985;27(5):335‐71. - PubMed
References to other published versions of this review
Nichol 2005
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

