Prevention of vincristine-induced peripheral neuropathy by genetic deletion of SARM1 in mice
- PMID: 27797810
- PMCID: PMC5840884
- DOI: 10.1093/brain/aww251
Prevention of vincristine-induced peripheral neuropathy by genetic deletion of SARM1 in mice
Abstract
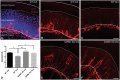
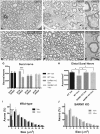
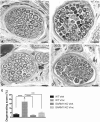
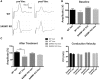
Peripheral polyneuropathy is a common and dose-limiting side effect of many important chemotherapeutic agents. Most such neuropathies are characterized by early axonal degeneration, yet therapies that inhibit this axonal destruction process do not currently exist. Recently, we and others discovered that genetic deletion of SARM1 (sterile alpha and TIR motif containing protein 1) dramatically protects axons from degeneration after axotomy in mice. This finding fuels hope that inhibition of SARM1 or its downstream components can be used therapeutically in patients threatened by axonal loss. However, axon loss in most neuropathies, including chemotherapy-induced peripheral neuropathy, is the result of subacute/chronic processes that may be regulated differently than the acute, one time insult of axotomy. Here we evaluate if genetic deletion of SARM1 decreases axonal degeneration in a mouse model of neuropathy induced by the chemotherapeutic agent vincristine. In wild-type mice, 4 weeks of twice-weekly intraperitoneal injections of 1.5 mg/kg vincristine cause pronounced mechanical and heat hyperalgesia, a significant decrease in tail compound nerve action potential amplitude, loss of intraepidermal nerve fibres and significant degeneration of myelinated axons in both the distal sural nerve and nerves of the toe. Neither the proximal sural nerve nor the motor tibial nerve exhibit axon loss. These findings are consistent with the development of a distal, sensory predominant axonal polyneuropathy that mimics vincristine-induced peripheral polyneuropathy in humans. Using the same regimen of vincristine treatment in SARM1 knockout mice, the development of mechanical and heat hyperalgesia is blocked and the loss in tail compound nerve action potential amplitude is prevented. Moreover, SARM1 knockout mice do not lose unmyelinated fibres in the skin or myelinated axons in the sural nerve and toe after vincristine. Hence, genetic deletion of SARM1 blocks the development of vincristine-induced peripheral polyneuropathy in mice. Our results reveal that subacute/chronic axon loss induced by vincristine occurs via a SARM1 mediated axonal destruction pathway, and that blocking this pathway prevents the development of vincristine-induced peripheral polyneuropathy. These findings, in conjunction with previous studies with axotomy and traumatic brain injury, establish SARM1 as the central determinant of a fundamental axonal degeneration pathway that is activated by diverse insults. We suggest that targeting SARM1 or its downstream effectors may be a viable therapeutic option to prevent vincristine-induced peripheral polyneuropathy and possibly other peripheral polyneuropathies.
Keywords: CIPN; SARM1; axonal degeneration; chemotherapy-induced peripheral neuropathy; vincristine.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Pharmacological SARM1 inhibition protects axon structure and function in paclitaxel-induced peripheral neuropathy.Brain. 2021 Nov 29;144(10):3226-3238. doi: 10.1093/brain/awab184. Brain. 2021. PMID: 33964142 Free PMC article.
-
Deletion of Sarm1 gene is neuroprotective in two models of peripheral neuropathy.J Peripher Nerv Syst. 2017 Sep;22(3):162-171. doi: 10.1111/jns.12219. J Peripher Nerv Syst. 2017. PMID: 28485482 Free PMC article.
-
Vincristine and bortezomib use distinct upstream mechanisms to activate a common SARM1-dependent axon degeneration program.JCI Insight. 2019 Sep 5;4(17):e129920. doi: 10.1172/jci.insight.129920. JCI Insight. 2019. PMID: 31484833 Free PMC article.
-
Lessons from Injury: How Nerve Injury Studies Reveal Basic Biological Mechanisms and Therapeutic Opportunities for Peripheral Nerve Diseases.Neurotherapeutics. 2021 Oct;18(4):2200-2221. doi: 10.1007/s13311-021-01125-3. Epub 2021 Sep 30. Neurotherapeutics. 2021. PMID: 34595734 Free PMC article. Review.
-
Axon degeneration: mechanistic insights lead to therapeutic opportunities for the prevention and treatment of peripheral neuropathy.Pain. 2019 May;160 Suppl 1(Suppl 1):S17-S22. doi: 10.1097/j.pain.0000000000001528. Pain. 2019. PMID: 31008845 Free PMC article. Review.
Cited by
-
Axon Biology in ALS: Mechanisms of Axon Degeneration and Prospects for Therapy.Neurotherapeutics. 2022 Jul;19(4):1133-1144. doi: 10.1007/s13311-022-01297-6. Epub 2022 Oct 7. Neurotherapeutics. 2022. PMID: 36207571 Free PMC article. Review.
-
Serum SARM1 Levels and Diabetic Peripheral Neuropathy in Type 2 Diabetes: Correlation with Clinical Neuropathy Scales and Nerve Conduction Studies and Impact of COVID-19 vaccination.Vaccines (Basel). 2024 Feb 17;12(2):209. doi: 10.3390/vaccines12020209. Vaccines (Basel). 2024. PMID: 38400192 Free PMC article.
-
Permeant fluorescent probes visualize the activation of SARM1 and uncover an anti-neurodegenerative drug candidate.Elife. 2021 May 4;10:e67381. doi: 10.7554/eLife.67381. Elife. 2021. PMID: 33944777 Free PMC article.
-
Single-Cell Analysis of Rohon-Beard Neurons Implicates Fgf Signaling in Axon Maintenance and Cell Survival.J Neurosci. 2024 Apr 17;44(16):e1600232024. doi: 10.1523/JNEUROSCI.1600-23.2024. J Neurosci. 2024. PMID: 38423763 Free PMC article.
-
Vitamin D3 Promotes Structural and Functional Recovery After Vincristine-Induced Peripheral Neuropathy in Rats: An Experimental Study.Cureus. 2023 Feb 14;15(2):e34979. doi: 10.7759/cureus.34979. eCollection 2023 Feb. Cureus. 2023. PMID: 36938210 Free PMC article.
References
-
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 2004; 305: 1010–3. - PubMed
-
- Authier N, Gillet J-P, Fialip J, Eschalier A, Coudore F. A new animal model of vincristine-induced nociceptive peripheral neuropathy. Neurotoxicology 2003; 24: 797–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

