The Organization of Mouse and Human Cortico-Hippocampal Networks Estimated by Intrinsic Functional Connectivity
- PMID: 27797832
- PMCID: PMC5193145
- DOI: 10.1093/cercor/bhw327
The Organization of Mouse and Human Cortico-Hippocampal Networks Estimated by Intrinsic Functional Connectivity
Abstract
While the hippocampal memory system has been relatively conserved across mammals, the cerebral cortex has undergone massive expansion. A central question in brain evolution is how cortical development affected the nature of cortical inputs to the hippocampus. To address this question, we compared cortico-hippocampal connectivity using intrinsic functional connectivity MRI (fcMRI) in awake mice and humans. We found that fcMRI recapitulates anatomical connectivity, demonstrating sensory mapping within the mouse parahippocampal region. Moreover, we identified a similar topographical modality-specific organization along the longitudinal axis of the mouse hippocampus, indicating that sensory information arriving at the hippocampus is only partly integrated. Finally, comparing cortico-hippocampal connectivity across species, we discovered preferential hippocampal connectivity of sensory cortical networks in mice compared with preferential connectivity of association cortical networks in humans. Supporting this observation in humans but not in mice, sensory and association cortical networks are connected to spatially distinct subregions within the parahippocampal region. Collectively, these findings indicate that sensory cortical networks are coupled to the mouse but not the human hippocampal memory system, suggesting that the emergence of expanded and new association areas in humans resulted in the rerouting of cortical information flow and dissociation of primary sensory cortices from the hippocampus.
Keywords: hippocampus; mammalian brain evolution; mouse connectivity atlas; mouse fMRI; resting state.
© The Author 2016. Published by Oxford University Press.
Figures

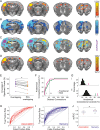

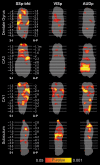
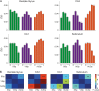

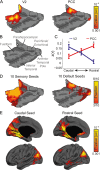
References
-
- Aronoff R, Matyas F, Mateo C, Ciron C, Schneider B, Petersen CCH. 2010. Long-range connectivity of mouse primary somatosensory barrel cortex. Eur J Neurosci. 31:2221–2233. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

