High hospital research participation and improved colorectal cancer survival outcomes: a population-based study
- PMID: 27797935
- PMCID: PMC5256392
- DOI: 10.1136/gutjnl-2015-311308
High hospital research participation and improved colorectal cancer survival outcomes: a population-based study
Abstract
Objective: In 2001, the National Institute for Health Research Cancer Research Network (NCRN) was established, leading to a rapid increase in clinical research activity across the English NHS. Using colorectal cancer (CRC) as an example, we test the hypothesis that high, sustained hospital-level participation in interventional clinical trials improves outcomes for all patients with CRC managed in those research-intensive hospitals.
Design: Data for patients diagnosed with CRC in England in 2001-2008 (n=209 968) were linked with data on accrual to NCRN CRC studies (n=30 998). Hospital Trusts were categorised by the proportion of patients accrued to interventional studies annually. Multivariable models investigated the relationship between 30-day postoperative mortality and 5-year survival and the level and duration of study participation.
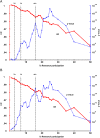
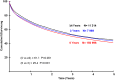
Results: Most of the Trusts achieving high participation were district general hospitals and the effects were not limited to cancer 'centres of excellence', although such centres do make substantial contributions. Patients treated in Trusts with high research participation (≥16%) in their year of diagnosis had lower postoperative mortality (p<0.001) and improved survival (p<0.001) after adjustment for casemix and hospital-level variables. The effects increased with sustained research participation, with a reduction in postoperative mortality of 1.5% (6.5%-5%, p<2.2×10-6) and an improvement in survival (p<10-19; 5-year difference: 3.8% (41.0%-44.8%)) comparing high participation for ≥4 years with 0 years.
Conclusions: There is a strong independent association between survival and participation in interventional clinical studies for all patients with CRC treated in the hospital study participants. Improvement precedes and increases with the level and years of sustained participation.
Keywords: CLINICAL TRIALS; COLORECTAL CANCER; HEALTH SERVICE RESEARCH.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
RK has received research funding from Bayer, Astra Zeneca and Glaxo Smith Kline (paid to the University of Leeds) and has been paid for a consulting or advisory role by Celldex Therapeutics. MS has received research funding from IntegraGen (paid to the University of Leeds). WG has been paid for a consulting or advisory role by Celgene and honoraria by Celgene.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
