IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer
- PMID: 27797936
- PMCID: PMC5406266
- DOI: 10.1136/gutjnl-2016-311585
IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer
Abstract
Objective: Limited efficacy of immune checkpoint inhibitors in pancreatic ductal adenocarcinoma (PDAC) has prompted investigation into combination therapy. We hypothesised that interleukin 6 (IL-6) blockade would modulate immunological features of PDAC and enhance the efficacy of anti-programmed death-1-ligand 1 (PD-L1) checkpoint inhibitor therapy.
Design: Transcription profiles and IL-6 secretion from primary patient-derived pancreatic stellate cells (PSCs) were analyzed via Nanostring and immunohistochemistry, respectively. In vivo efficacy and mechanistic studies were conducted with antibodies (Abs) targeting IL-6, PD-L1, CD4 or CD8 in subcutaneous or orthotopic models using Panc02, MT5 or KPC-luc cell lines; and the aggressive, genetically engineered PDAC model (KrasLSL-G12D, Trp53LSL-R270H, Pdx1-cre, Brca2F/F (KPC-Brca2 mice)). Systemic and local changes in immunophenotype were measured by flow cytometry or immunohistochemical analysis.
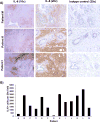
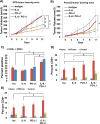
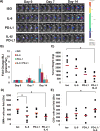
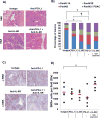
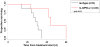
Results: PSCs (n=12) demonstrated prominent IL-6 expression, which was localised to stroma of tumours. Combined IL-6 and PD-L1 blockade elicited efficacy in mice bearing subcutaneous MT5 (p<0.02) and Panc02 tumours (p=0.046), which was accompanied by increased intratumoural effector T lymphocytes (CD62L-CD44-). CD8-depleting but not CD4-depleting Abs abrogated the efficacy of combined IL-6 and PD-L1 blockade in mice bearing Panc02 tumours (p=0.0016). This treatment combination also elicited significant antitumour activity in mice bearing orthotopic KPC-luc tumours and limited tumour progression in KPC-Brca2 mice (p<0.001). Histological analysis revealed increased T-cell infiltration and reduced α-smooth muscle actin cells in tumours from multiple models. Finally, IL-6 and PD-L1 blockade increased overall survival in KPC-Brca2 mice compared with isotype controls (p=0.0012).
Conclusions: These preclinical results indicate that targeted inhibition of IL-6 may enhance the efficacy of anti-PD-L1 in PDAC.
Keywords: IMMUNOTHERAPY; INTERLEUKINS; PANCREATIC CANCER.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: None declared.
Figures




References
-
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. - PubMed
-
- Lesina M, Kurkowski MU, Ludes K, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
