OPTIMUM: a protocol for a multicentre randomised controlled trial comparing Out Patient Talc slurry via Indwelling pleural catheter for Malignant pleural effusion vs Usual inpatient Management
- PMID: 27798020
- PMCID: PMC5073842
- DOI: 10.1136/bmjopen-2016-012795
OPTIMUM: a protocol for a multicentre randomised controlled trial comparing Out Patient Talc slurry via Indwelling pleural catheter for Malignant pleural effusion vs Usual inpatient Management
Erratum in
-
Correction.BMJ Open. 2016 Nov 14;6(11):e012795corr1. doi: 10.1136/bmjopen-2016-012795corr1. BMJ Open. 2016. PMID: 28186953 Free PMC article. No abstract available.
Abstract
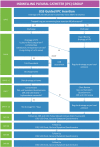
Introduction: The development of malignant pleural effusion (MPE) results in disabling breathlessness, pain and reduced physical capability with treatment a palliative strategy. Ambulatory management of MPE has the potential to improve quality of life (QoL). The OPTIMUM trial is designed to determine whether full outpatient management of MPE with an indwelling pleural catheter (IPC) and pleurodesis improves QoL compared with traditional inpatient care with a chest drain and talc pleurodesis. OPTIMUM is currently open for any centres interested in collaborating in this study.
Methods and analysis: OPTIMUM is a multicentre non-blinded randomised controlled trial. Patients with a diagnosis of MPE will be identified and screened for eligibility. Consenting participants will be randomised 1:1 either to an outpatient ambulatory pathway using IPCs and talc pleurodesis or standard inpatient treatment with chest drain and talc pleurodesis as per British Thoracic Society guidelines. The primary outcome measure is global health-related QoL at 30 days measured using the EORTC QLQ-C30 questionnaire. Secondary outcome measures include breathlessness and pain measured using a 100 mm Visual Analogue Scale and health-related QoL at 60 and 90 days. A sample size of 142 patients is needed to demonstrate a clinically significant difference of 8 points in global health status at 30 days, for an 80% power and a 5% significance level.
Ethics and dissemination: The study has been approved by the NRES Committee South East Coast-Brighton and Sussex (reference 15/LO/1018). The trial results will be published in peer-reviewed journals and presented at scientific conferences.
Trial registration numbers: UKCRN19615 and ISRCTN15503522; Pre-results.
Keywords: Indwelling pleural catheter; Malignant pleural effusion; Quality of life.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
