China PEACE risk estimation tool for in-hospital death from acute myocardial infarction: an early risk classification tree for decisions about fibrinolytic therapy
- PMID: 27798032
- PMCID: PMC5093680
- DOI: 10.1136/bmjopen-2016-013355
China PEACE risk estimation tool for in-hospital death from acute myocardial infarction: an early risk classification tree for decisions about fibrinolytic therapy
Abstract
Objectives: As the predominant approach to acute reperfusion for ST segment elevation myocardial infarction (STEMI) in many countries, fibrinolytic therapy provides a relative risk reduction for death of ∼16% across the range of baseline risk. For patients with low baseline mortality risk, fibrinolytic therapy may therefore provide little benefit, which may be offset by the risk of major bleeding. We aimed to construct a tool to determine if it is possible to identify a low-risk group among fibrinolytic therapy-eligible patients.
Design: Cross-sectional study.
Setting: The China Patient-centered Evaluative Assessment of Cardiac Events (PEACE) study includes a nationally representative retrospective sample of patients admitted with acute myocardial infarction (AMI) in 162 hospitals.
Participants: 3741 patients with STEMI who were fibrinolytic-eligible but did not receive reperfusion therapy.
Main outcome measures: In-hospital mortality, which was defined as a composite of death occurring within hospitalisation or withdrawal from treatment due to a terminal status at discharge.
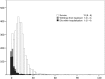
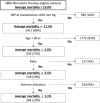
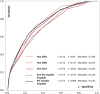
Results: In the study cohort, the in-hospital mortality was 14.7%. In the derivation cohort and the validation cohort, the combination of systolic blood pressure (≥100 mm Hg), age (<60 years old) and gender (male) identified one-fifth of the cohort with an average mortality rate of <3.0%. Half of this low risk group-those with non-anterior AMI-had an average in-hospital death risk of 1.5%.
Conclusions: Nearly, one in five patients with STEMI who are eligible for fibrinolytic therapy are at a low risk for in-hospital death. Three simple factors available at the time of presentation can identify these individuals and support decision-making about the use of fibrinolytic therapy.
Trial registration number: NCT01624883.
Keywords: Fibrinolytic therapy; risk tool.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
HMK reports research grants from Medtronic and Johnson & Johnson, through Yale University, to develop methods of clinical trial data sharing, and the chair of a cardiac scientific advisory board for UnitedHealth.
Figures

References
-
- Chen Y, Jiang L, Zhang Q et al. . Doctor-reported hospital management of acute coronary syndrome in China: a nationwide survey of 1029 hospitals in 30 provinces. World J Cardiovasc Dis 2012;2:168–76.
-
- Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet 1994;343:311–22. - PubMed
-
- O'Gara PT, Kushner FG, Ascheim DD et al. , American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:e78–e140. 10.1016/j.jacc.2012.11.019 - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
