Computational modelling for congenital heart disease: how far are we from clinical translation?
- PMID: 27798056
- PMCID: PMC5284484
- DOI: 10.1136/heartjnl-2016-310423
Computational modelling for congenital heart disease: how far are we from clinical translation?
Abstract
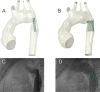
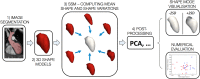
Computational models of congenital heart disease (CHD) have become increasingly sophisticated over the last 20 years. They can provide an insight into complex flow phenomena, allow for testing devices into patient-specific anatomies (pre-CHD or post-CHD repair) and generate predictive data. This has been applied to different CHD scenarios, including patients with single ventricle, tetralogy of Fallot, aortic coarctation and transposition of the great arteries. Patient-specific simulations have been shown to be informative for preprocedural planning in complex cases, allowing for virtual stent deployment. Novel techniques such as statistical shape modelling can further aid in the morphological assessment of CHD, risk stratification of patients and possible identification of new 'shape biomarkers'. Cardiovascular statistical shape models can provide valuable insights into phenomena such as ventricular growth in tetralogy of Fallot, or morphological aortic arch differences in repaired coarctation. In a constant move towards more realistic simulations, models can also account for multiscale phenomena (eg, thrombus formation) and importantly include measures of uncertainty (ie, CIs around simulation results). While their potential to aid understanding of CHD, surgical/procedural decision-making and personalisation of treatments is undeniable, important elements are still lacking prior to clinical translation of computational models in the field of CHD, that is, large validation studies, cost-effectiveness evaluation and establishing possible improvements in patient outcomes.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- Silva Vieira M, Hussain T, Figueroa CA. Patient-specific image-based computational modeling in congenital heart disease: a clinician perspective. J Cardiol Ther 2015;2:436–48. 10.17554/j.issn.2309-6861.2015.02.96 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
