Variegated yet non-random rod and cone photoreceptor disease patterns in RPGR-ORF15-associated retinal degeneration
- PMID: 27798110
- PMCID: PMC6078602
- DOI: 10.1093/hmg/ddw361
Variegated yet non-random rod and cone photoreceptor disease patterns in RPGR-ORF15-associated retinal degeneration
Erratum in
-
Variegated yet non-random rod and cone photoreceptor disease patterns in RPGR-ORF15-associated retinal degeneration.Hum Mol Genet. 2019 Jan 1;28(1):175. doi: 10.1093/hmg/ddy342. Hum Mol Genet. 2019. PMID: 30285110 Free PMC article. No abstract available.
Abstract
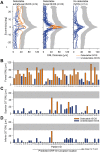
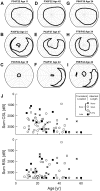
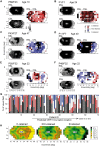
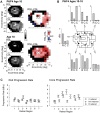
Mutations in the ORF15 exon of the RPGR gene cause a common form of X-linked retinitis pigmentosa, which often results in severe loss of vision. In dogs and mice, gene augmentation therapy has been shown to arrest the progressive degeneration of rod and cone photoreceptors. However, the distribution of potentially treatable photoreceptors across the human retinas and the rate of degeneration are not known. Here, we have defined structural and functional features of the disease in 70 individuals with ORF15 mutations. We also correlated the features observed in patients with those of three Rpgr-mutant (Rpgr-ko, Rd9, and Rpgr-cko) mice. In patients, there was pronounced macular disease. Across the retina, rod and cone dysfunction showed a range of patterns and a spectrum of severity between individuals, but a high symmetry was observed between eyes of each individual. Genotype was not related to disease expression. In the Rpgr-ko mice, there were intra-retinal differences in rhodopsin and cone opsin trafficking. In Rd9 and Rpgr-cko mice, retinal degeneration showed inter-ocular symmetry. Longitudinal results in patients revealed localized rod and cone dysfunction with progression rates of 0.8 to 1.3 log per decade in sensitivity loss. Relatively retained rod and cone photoreceptors in mid- and far-peripheral temporal-inferior and nasal-inferior visual field regions should be good targets for future localized gene therapies in patients.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
RPGR-associated retinal degeneration in human X-linked RP and a murine model.Invest Ophthalmol Vis Sci. 2012 Aug 15;53(9):5594-608. doi: 10.1167/iovs.12-10070. Invest Ophthalmol Vis Sci. 2012. PMID: 22807293 Free PMC article.
-
Rd9 is a naturally occurring mouse model of a common form of retinitis pigmentosa caused by mutations in RPGR-ORF15.PLoS One. 2012;7(5):e35865. doi: 10.1371/journal.pone.0035865. Epub 2012 May 1. PLoS One. 2012. PMID: 22563472 Free PMC article.
-
Cone versus rod disease in a mutant Rpgr mouse caused by different genetic backgrounds.Invest Ophthalmol Vis Sci. 2010 Feb;51(2):1106-15. doi: 10.1167/iovs.08-2742. Epub 2009 Dec 10. Invest Ophthalmol Vis Sci. 2010. PMID: 20007830
-
PRCD Is a Small Disc-Specific Rhodopsin-Binding Protein of Unknown Function.Adv Exp Med Biol. 2019;1185:531-535. doi: 10.1007/978-3-030-27378-1_87. Adv Exp Med Biol. 2019. PMID: 31884666 Review.
-
More Than Meets the Eye: Current Understanding of RPGR Function.Adv Exp Med Biol. 2018;1074:521-538. doi: 10.1007/978-3-319-75402-4_64. Adv Exp Med Biol. 2018. PMID: 29721984 Review.
Cited by
-
Optical Coherence Tomography Angiography of RPGR-Associated Retinitis Pigmentosa Suggests Foveal Avascular Zone is a Biomarker for Vision Loss.Ophthalmic Surg Lasers Imaging Retina. 2019 Feb 1;50(2):e44-e48. doi: 10.3928/23258160-20190129-18. Ophthalmic Surg Lasers Imaging Retina. 2019. PMID: 30768229 Free PMC article.
-
Foveal Therapy in Blue Cone Monochromacy: Predictions of Visual Potential From Artificial Intelligence.Front Neurosci. 2020 Aug 3;14:800. doi: 10.3389/fnins.2020.00800. eCollection 2020. Front Neurosci. 2020. PMID: 32848570 Free PMC article.
-
Toxicity and Efficacy Evaluation of an Adeno-Associated Virus Vector Expressing Codon-Optimized RPGR Delivered by Subretinal Injection in a Canine Model of X-linked Retinitis Pigmentosa.Hum Gene Ther. 2020 Feb;31(3-4):253-267. doi: 10.1089/hum.2019.297. Epub 2020 Feb 6. Hum Gene Ther. 2020. PMID: 31910043 Free PMC article.
-
RPGR-Associated Dystrophies: Clinical, Genetic, and Histopathological Features.Int J Mol Sci. 2020 Jan 28;21(3):835. doi: 10.3390/ijms21030835. Int J Mol Sci. 2020. PMID: 32012938 Free PMC article.
-
Photoreceptor Disease at Ambiguous Transition Zones in Inherited Retinal Degenerations.Transl Vis Sci Technol. 2025 Aug 1;14(8):11. doi: 10.1167/tvst.14.8.11. Transl Vis Sci Technol. 2025. PMID: 40762536 Free PMC article.
References
-
- Bramall A.N., Wright A.F., Jacobson S.G., McInnes R.R. (2010) The genomic, biochemical, and cellular responses of the retina in inherited photoreceptor degenerations and prospects for the treatment of these disorders. Annu. Rev. Neurosci., 33, 441–472. - PubMed
-
- Wright A.F., Chakarova C.F., Abd El-Aziz M.M., Bhattacharya S.S. (2010) Photoreceptor degeneration, genetic and mechanistic dissection of a complex trait. Nat. Rev. Genet., 11, 273–284. - PubMed
-
- Vervoort R., Lennon A., Bird A.C., Tulloch B., Axton R., Miano M.G., Meindl A., Meitinger T., Ciccodicola A., Wright A.F. (2000) Mutational hot spot within a new RPGR exon in X-linked retinitis pigmentosa. Nat. Genet., 25, 462–466. - PubMed
-
- Breuer D.K., Yashar B.M., Filippova E., Hiriyanna S., Lyons R.H., Mears A.J., Asaye B., Acar C., Vervoort R., Wright A.F., et al. (2002) A comprehensive mutation analysis of RP2 and RPGR in a North American cohort of families with X-linked retinitis pigmentosa. Am. J. Hum. Genet., 70, 1545–1554. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

