Conditional Deletion of the L-Type Calcium Channel Cav1.2 in Oligodendrocyte Progenitor Cells Affects Postnatal Myelination in Mice
- PMID: 27798140
- PMCID: PMC5083013
- DOI: 10.1523/JNEUROSCI.1770-16.2016
Conditional Deletion of the L-Type Calcium Channel Cav1.2 in Oligodendrocyte Progenitor Cells Affects Postnatal Myelination in Mice
Abstract
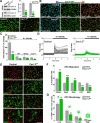
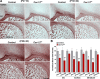
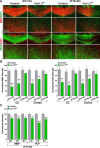
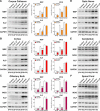
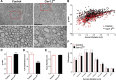
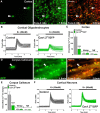
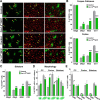
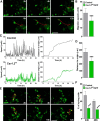
To determine whether L-type voltage-operated Ca2+ channels (L-VOCCs) are required for oligodendrocyte progenitor cell (OPC) development, we generated an inducible conditional knock-out mouse in which the L-VOCC isoform Cav1.2 was postnatally deleted in NG2-positive OPCs. A significant hypomyelination was found in the brains of the Cav1.2 conditional knock-out (Cav1.2KO) mice specifically when the Cav1.2 deletion was induced in OPCs during the first 2 postnatal weeks. A decrease in myelin proteins expression was visible in several brain structures, including the corpus callosum, cortex, and striatum, and the corpus callosum of Cav1.2KO animals showed an important decrease in the percentage of myelinated axons and a substantial increase in the mean g-ratio of myelinated axons. The reduced myelination was accompanied by an important decline in the number of myelinating oligodendrocytes and in the rate of OPC proliferation. Furthermore, using a triple transgenic mouse in which all of the Cav1.2KO OPCs were tracked by a Cre reporter, we found that Cav1.2KO OPCs produce less mature oligodendrocytes than control cells. Finally, live-cell imaging in early postnatal brain slices revealed that the migration and proliferation of subventricular zone OPCs is decreased in the Cav1.2KO mice. These results indicate that the L-VOCC isoform Cav1.2 modulates oligodendrocyte development and suggest that Ca2+ influx mediated by L-VOCCs in OPCs is necessary for normal myelination.
Significance statement: Overall, it is clear that cells in the oligodendrocyte lineage exhibit remarkable plasticity with regard to the expression of Ca2+ channels and that perturbation of Ca2+ homeostasis likely plays an important role in the pathogenesis underlying demyelinating diseases. To determine whether voltage-gated Ca2+ entry is involved in oligodendrocyte maturation and myelination, we used a conditional knock-out mouse for voltage-operated Ca2+ channels in oligodendrocyte progenitor cells. Our results indicate that voltage-operated Ca2+ channels can modulate oligodendrocyte development in the postnatal brain and suggest that voltage-gated Ca2+ influx in oligodendroglial cells is critical for normal myelination. These findings could lead to novel approaches to intervene in neurodegenerative diseases in which myelin is lost or damaged.
Keywords: Cav1.2; calcium; myelination; oligodendrocytes; voltage-operated Ca2+ channels.
Copyright © 2016 the authors 0270-6474/16/3610853-17$15.00/0.
Figures


Comment in
-
Cytoskeletal Regulation of Oligodendrocyte Differentiation and Myelination.J Neurosci. 2017 Aug 16;37(33):7797-7799. doi: 10.1523/JNEUROSCI.1398-17.2017. J Neurosci. 2017. PMID: 28821599 Free PMC article. No abstract available.
References
-
- Akopian G, Kressin K, Derouiche A, Steinhäuser C. Identified glial cells in the early postnatal mouse hippocampus display different types of Ca++ currents. Glia. 1996;17:181–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
