In Utero Exposure to Valproic Acid Induces Neocortical Dysgenesis via Dysregulation of Neural Progenitor Cell Proliferation/Differentiation
- PMID: 27798144
- PMCID: PMC6601887
- DOI: 10.1523/JNEUROSCI.0229-16.2016
In Utero Exposure to Valproic Acid Induces Neocortical Dysgenesis via Dysregulation of Neural Progenitor Cell Proliferation/Differentiation
Abstract
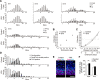
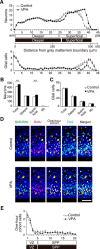
Valproic acid (VPA), a widely used antiepileptic drug, is an inhibitor of histone deacetylases, which epigenetically modify cell proliferation/differentiation in developing tissues. A series of recent clinical studies in humans reported that VPA exposure in utero impaired histogenesis and the development of the central nervous system, leading to increased risks of congenital malformation and the impairment of higher brain functions in children. In the present study conducted in mice, we report that VPA exposure in utero (1) increases the amount of acetylated histone proteins, (2) alters the expression of G1-phase regulatory proteins, (3) inhibits the cell cycle exit of neural progenitor cells during the early stage of neocortical histogenesis, and (4) increases the production of projection neurons distributed in the superficial neocortical layers in embryonic brains. Together, our findings show that VPA exposure in utero alters proliferation/differentiation characteristics of neural progenitor cells and hence leads to the neocortical dysgenesis.
Significance statement: This study provides new insight into the mechanisms of how an altered in utero environment, such as drug exposure, affects the generation of neurons prenatally. The antiepileptic drug valproic acid (VPA) is a good target molecule as in utero exposure to VPA has been repeatedly reported to increase the risk of nervous system malformations and to impair higher brain functions in children. We show that VPA decreases the probability of differentiation of the neural progenitor cells (NPCs) in mice, resulting in an abnormally increased number of projection neurons in the superficial layers of the neocortex. Further, we suggest that histone deacetylase inhibition by VPA may be involved in the dysregulation of proliferation/differentiation characteristics of NPCs.
Keywords: deacetylation; histone; neocortex; neural progenitor cell; neuronogenesis.
Copyright © 2016 the authors 0270-6474/16/3610908-12$15.00/0.
Figures




References
-
- Bromley RL, Mawer GE, Briggs M, Cheyne C, Clayton-Smith J, García-Fiñana M, Kneen R, Lucas SB, Shallcross R, Baker GA. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2013;84:637–643. doi: 10.1136/jnnp-2012-304270. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
