WNK-Cab39-NKCC1 signaling increases the susceptibility to ischemic brain damage in hypertensive rats
- PMID: 27798271
- PMCID: PMC5536788
- DOI: 10.1177/0271678X16675368
WNK-Cab39-NKCC1 signaling increases the susceptibility to ischemic brain damage in hypertensive rats
Abstract
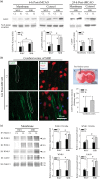
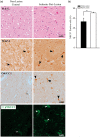
With-no-lysine kinase (WNK) and Na+-K+-2Cl- cotransporter 1 (NKCC1) are involved in the pathogenesis of hypertension. In this study, we investigated the WNK-NKCC1 signaling pathway in spontaneously hypertensive rats (SHR) and their associated susceptibility to stroke injury. Basal NKCC1 protein levels were higher in SHR than in normotensive Wistar Kyoto (WKY) rat brains. After inducing ischemic stroke, adult male WKY and SHR received either saline or NKCC1 inhibitor bumetanide (10 mg/kg/day, i.p.) starting at 3-h post-reperfusion. NKCC1 inhibition blunted the extent of ischemic infarction in SHR and improved their neurobehavioral functions. Interestingly, ischemia led to increased NKCC1 phosphorylation in SHR but not in WKY rats. Pronounced elevation of WNK1, WNK2 and WNK4 protein and downregulation of WNK3 were detected in ischemic SHR, but not in ischemic WKY rats. Upregulation of WNK-NKCC1 complex in ischemic SHR brain was associated with increased Ca2+-binding protein 39 (Cab39), without increases in Ste20-related proline alanine-rich kinase or oxidative stress-responsive kinase-1. Moreover, subacute middle cerebral artery stroke human brain autopsy exhibited increased expression of NKCC1 protein. We conclude that augmented WNK-Cab39-NKCC1 signaling in SHR is associated with an increased susceptibility to ischemic brain damage and may serve as a novel target for anti-hypertensive and anti-ischemic stroke therapy.
Keywords: Bumetanide; Cab39; NKCC1; SHR; WNK kinase; hypertension; ischemic stroke.
Figures





References
-
- Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the wnk-spak/osr1 signalling pathway. J Cell Sci 2008; 121: 3293–3304. - PubMed
-
- Wilson FH, Disse-Nicodeme S, Choate KA, et al. Human hypertension caused by mutations in wnk kinases. Science 2001; 293: 1107–1112. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

