Growth at 2 Years of Age in HIV-exposed Uninfected Children in the United States by Trimester of Maternal Antiretroviral Initiation
- PMID: 27798548
- PMCID: PMC5526594
- DOI: 10.1097/INF.0000000000001387
Growth at 2 Years of Age in HIV-exposed Uninfected Children in the United States by Trimester of Maternal Antiretroviral Initiation
Abstract
Background: Abnormal childhood growth may affect future health. Maternal tenofovir (TFV) use was associated with lower body length and head circumference at 1 year of age in HIV-exposed uninfected (HEU) US children.
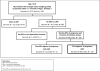
Methods: We studied 509 HEU children in the US-based Surveillance Monitoring of Antiretroviral Therapy Toxicities cohort whose HIV-infected mothers were not using antiretrovirals at the last menstrual period and began combination antiretroviral therapy (cART) in pregnancy (cART initiators). We examined adjusted associations between antiretrovirals and Centers for Disease Control 2000 growth Z scores at 2 years of age within trimester of cART initiation: weight (weight Z score), length (length Z score), weight-for-length [weight-for-length Z score (WFLZ)], triceps skinfold Z score (TSFZ) and head circumference (head circumference Z score).
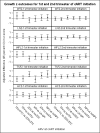
Results: Mothers mean age was 28.6 years; 57% were black non-Hispanic and 19% delivered at <37 weeks gestation. At 2 years, mean weight Z score, length Z score, WFLZ and head circumference Z score were above average (P < 0.05), whereas TSFZ (P = 0.57) did not differ from average. WFLZ was >1.64 standard deviation (SD) (>95th percentile) in 13%. Among children of first-trimester cART initiators, TFV+emtricitabine-exposed children had slightly higher mean WFLZ (0.45 SD; 95% confidence interval: -0.10 to 1.00) and lower TSFZ (-0.55 SD; 95% confidence interval: -1.07 to -0.02) compared with zidovudine+lamivudine-exposed children. TSFZ was lower in those exposed to boosted protease inhibitors. In contrast, growth in children of second trimester cART initiators did not differ by antiretroviral exposures.
Conclusion: Growth was above average in HEU; 13% were obese. Maternal TFV use was not associated with lower length or head circumference at 2 years of age, as hypothesized, but may be related to greater weight among those exposed to cART early in pregnancy.
Conflict of interest statement
Figures
References
-
- WHO. World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: toward universal access (2010 version) - PubMed
-
- Jauniaux E, Nessmann C, Imbert MC, Meuris S, Puissant F, Hustin J. Morphological aspects of the placenta in HIV pregnancies. Placenta. 1988;9(6):633–42. - PubMed
-
- Vermaak A, Theron GB, Schubert PT, et al. Morphologic changes in the placentas of HIV-positive women and their association with degree of immune suppression. Int J of Gynaecol Obs. 2012;119(3):239–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

