Microcins mediate competition among Enterobacteriaceae in the inflamed gut
- PMID: 27798599
- PMCID: PMC5145735
- DOI: 10.1038/nature20557
Microcins mediate competition among Enterobacteriaceae in the inflamed gut
Abstract
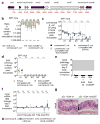
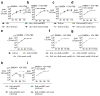
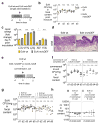
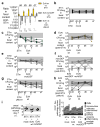
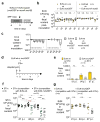
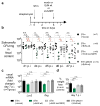
The Enterobacteriaceae are a family of Gram-negative bacteria that include commensal organisms as well as primary and opportunistic pathogens that are among the leading causes of morbidity and mortality worldwide. Although Enterobacteriaceae often comprise less than 1% of a healthy intestine's microbiota, some of these organisms can bloom in the inflamed gut; expansion of enterobacteria is a hallmark of microbial imbalance known as dysbiosis. Microcins are small secreted proteins that possess antimicrobial activity in vitro, but whose role in vivo has been unclear. Here we demonstrate that microcins enable the probiotic bacterium Escherichia coli Nissle 1917 (EcN) to limit the expansion of competing Enterobacteriaceae (including pathogens and pathobionts) during intestinal inflammation. Microcin-producing EcN limits the growth of competitors in the inflamed intestine, including commensal E. coli, adherent-invasive E. coli and the related pathogen Salmonella enterica. Moreover, only therapeutic administration of the wild-type, microcin-producing EcN to mice previously infected with S. enterica substantially reduced intestinal colonization by the pathogen. Our work provides the first evidence that microcins mediate inter- and intraspecies competition among the Enterobacteriaceae in the inflamed gut. Moreover, we show that microcins can act as narrow-spectrum therapeutics to inhibit enteric pathogens and reduce enterobacterial blooms.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures








References
-
- Stecher B. The Roles of Inflammation, Nutrient Availability and the Commensal Microbiota in Enteric Pathogen Infection. Microbiol Spectr. 2015;3 - PubMed

