Fast volumetric calcium imaging across multiple cortical layers using sculpted light
- PMID: 27798612
- PMCID: PMC5531274
- DOI: 10.1038/nmeth.4040
Fast volumetric calcium imaging across multiple cortical layers using sculpted light
Abstract
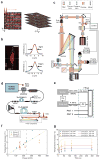
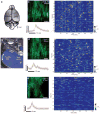
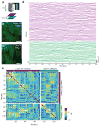
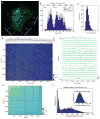
Although whole-organism calcium imaging in small and semi-transparent animals has been demonstrated, capturing the functional dynamics of large-scale neuronal circuits in awake behaving mammals at high speed and resolution has remained one of the main frontiers in systems neuroscience. Here we present a method based on light sculpting that enables unbiased single- and dual-plane high-speed (up to 160 Hz) calcium imaging as well as in vivo volumetric calcium imaging of a mouse cortical column (0.5 mm × 0.5 mm × 0.5 mm) at single-cell resolution and fast volume rates (3-6 Hz). We achieved this by tailoring the point-spread function of our microscope to the structures of interest while maximizing the signal-to-noise ratio using a home-built fiber laser amplifier with pulses that are synchronized to the imaging voxel speed. This enabled in vivo recording of calcium dynamics of several thousand neurons across cortical layers and in the hippocampus of awake behaving mice.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EH. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science. 2004;305:1007–1009. - PubMed
-
- Levoy M, Ng R, Adams A, Footer M, Horowitz M. Light field microscopy. Acm Transactions on Graphics. 2006;25:924–934. doi: 10.1145/1141911.1141976. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

