Prospective, Multicentre, Nationwide Clinical Data from 600 Cases of Acute Pancreatitis
- PMID: 27798670
- PMCID: PMC5087847
- DOI: 10.1371/journal.pone.0165309
Prospective, Multicentre, Nationwide Clinical Data from 600 Cases of Acute Pancreatitis
Abstract
Objective: The aim of this study was to analyse the clinical characteristics of acute pancreatitis (AP) in a prospectively collected, large, multicentre cohort and to validate the major recommendations in the IAP/APA evidence-based guidelines for the management of AP.
Design: Eighty-six different clinical parameters were collected using an electronic clinical research form designed by the Hungarian Pancreatic Study Group.
Patients: 600 adult patients diagnosed with AP were prospectively enrolled from 17 Hungarian centres over a two-year period from 1 January 2013.
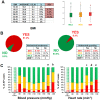
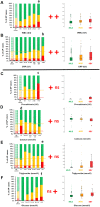
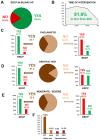
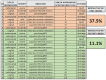
Main results: With respect to aetiology, biliary and alcoholic pancreatitis represented the two most common forms of AP. The prevalence of biliary AP was higher in women, whereas alcoholic AP was more common in men. Hyperlipidaemia was a risk factor for severity, lack of serum enzyme elevation posed a risk for severe AP, and lack of abdominal pain at admission demonstrated a risk for mortality. Abdominal tenderness developed in all the patients with severe AP, while lack of abdominal tenderness was a favourable sign for mortality. Importantly, lung injury at admission was associated with mortality. With regard to laboratory parameters, white blood cell count and CRP were the two most sensitive indicators for severe AP. The most common local complication was peripancreatic fluid, whereas the most common distant organ failure in severe AP was lung injury. Deviation from the recommendations in the IAP/APA evidence-based guidelines on fluid replacement, enteral nutrition and timing of interventions increased severity and mortality.
Conclusions: Analysis of a large, nationwide, prospective cohort of AP cases allowed for the identification of important determinants of severity and mortality. Evidence-based guidelines should be observed rigorously to improve outcomes in AP.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Nawaz H, Mounzer R, Yadav D, Yabes JG, Slivka A, Whitcomb DC, et al. Revised Atlanta and determinant-based classification: application in a prospective cohort of acute pancreatitis patients. Am J Gastroenterol. 2013;108(12):1911–7. Epub 2013/10/16. 10.1038/ajg.2013.348 ajg2013348 [pii]. . - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

