Awareness, Knowledge, and Perceptions of Biosimilars Among Specialty Physicians
- PMID: 27798772
- PMCID: PMC5126187
- DOI: 10.1007/s12325-016-0431-5
Awareness, Knowledge, and Perceptions of Biosimilars Among Specialty Physicians
Abstract
Introduction: The Biosimilars Forum conducted a survey through an independent organization from November 20, 2015 to January 4, 2016 in order to assess current levels of awareness, knowledge, and perceptions of biosimilars among US specialty physicians who already prescribe biologics. The survey was intended to provide a baseline level of knowledge about biosimilars and will be repeated in 2-3 years in order to monitor trends over time.
Methods: A 19-question survey was created by the Biosimilars Forum and was administered by an independent third party.
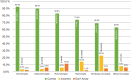
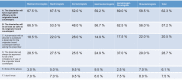
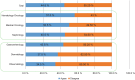
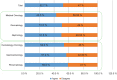
Results: Responses were obtained from 1201 US physicians across specialties that are high prescribers of biologics, including dermatologists, gastroenterologists, hematologist-oncologists, medical oncologists, nephrologists, and rheumatologists.
Conclusions: The results of this survey highlight a significant need for evidence-based education about biosimilars for physicians across specialties. Five major knowledge gaps were identified: defining biologics, biosimilars, and biosimilarity; understanding the approval process and the use of "totality of evidence" to evaluate biosimilars; understanding that the safety and immunogenicity of a biosimilar are comparable to the originator biologic; understanding the rationale for extrapolation of indications; and defining interchangeability and the related rules regarding pharmacy-level substitution.
Funding: Biosimilars Forum.
Keywords: Biologic; Biosimilar; Biosimilarity; Extrapolation; Immunogenicity; Interchangeability; Originator biologic.
Figures




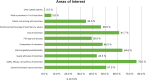
References
-
- US Food and Drug Administration. FDA approves first biosimilar product Zarxio. Retrieved from: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm436648.htm. Accessed 6 Mar 2015.
-
- US Food and Drug Administration. FDA approves Inflectra, a biosimilar to Remicade. Retrieved from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494227.htm. Accessed 5 Apr 2016.
-
- US Food and Drug Administration. FDA approved Erelzi, a biosimilar to Enbrel. Retrieved from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518639.htm. Accessed 30 Aug 2016.
-
- Eastern Research Group, Inc. Review of biosimilar product applications: study of workload volume and full costs. Retrieved from: http://www.fda.gov/downloads/ForIndustry/UserFees/BiosimilarUserFeeActBs.... Accessed 24 Feb 2016.
-
- IMS Health. The Impact of Biosimilar Competition. Retrieved from: http://ec.europa.eu/growth/tools-databases/newsroom/cf/itemdetail.cfm?it.... Accessed 20 June 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

