Vitronectin Binds to a Specific Stretch within the Head Region of Yersinia Adhesin A and Thereby Modulates Yersinia enterocolitica Host Interaction
- PMID: 27798934
- PMCID: PMC6738804
- DOI: 10.1159/000449200
Vitronectin Binds to a Specific Stretch within the Head Region of Yersinia Adhesin A and Thereby Modulates Yersinia enterocolitica Host Interaction
Abstract
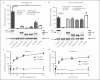
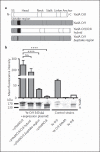
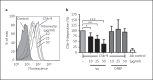
Complement resistance is an important virulence trait of Yersinia enterocolitica (Ye). The predominant virulence factor expressed by Ye is Yersinia adhesin A (YadA), which enables bacterial attachment to host cells and extracellular matrix and additionally allows the acquisition of soluble serum factors. The serum glycoprotein vitronectin (Vn) acts as an inhibitory regulator of the terminal complement complex by inhibiting the lytic pore formation. Here, we show YadA-mediated direct interaction of Ye with Vn and investigated the role of this Vn binding during mouse infection in vivo. Using different Yersinia strains, we identified a short stretch in the YadA head domain of Ye O:9 E40, similar to the 'uptake region' of Y. pseudotuberculosis YPIII YadA, as crucial for efficient Vn binding. Using recombinant fragments of Vn, we found the C-terminal part of Vn, including heparin-binding domain 3, to be responsible for binding to YadA. Moreover, we found that Vn bound to the bacterial surface is still functionally active and thus inhibits C5b-9 formation. In a mouse infection model, we demonstrate that Vn reduces complement-mediated killing of Ye O:9 E40 and, thus, improved bacterial survival. Taken together, these findings show that YadA-mediated Vn binding influences Ye pathogenesis.
© 2016 S. Karger AG, Basel.
Figures

References
-
- Bottone EJ. Yersinia enterocolitica: overview and epidemiologic correlates. Microbes Infect. 1999;1:323–333. - PubMed
-
- Cover TL, Aber RC. Yersinia enterocolitica. N Engl J Med. 1989;321:16–24. - PubMed
-
- Isberg RR. Mammalian cell adhesion functions and cellular penetration of enteropathogenic Yersinia species. Mol Microbiol. 1989;3:1449–1453. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

