Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles
- PMID: 27799072
- PMCID: PMC5508927
- DOI: 10.1186/s12987-016-0040-3
Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles
Abstract
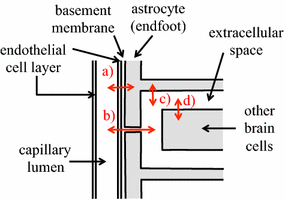
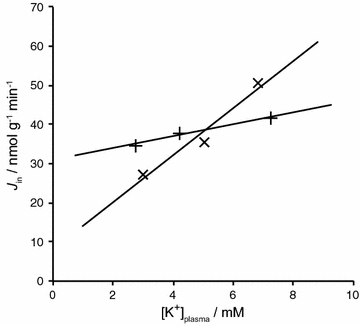
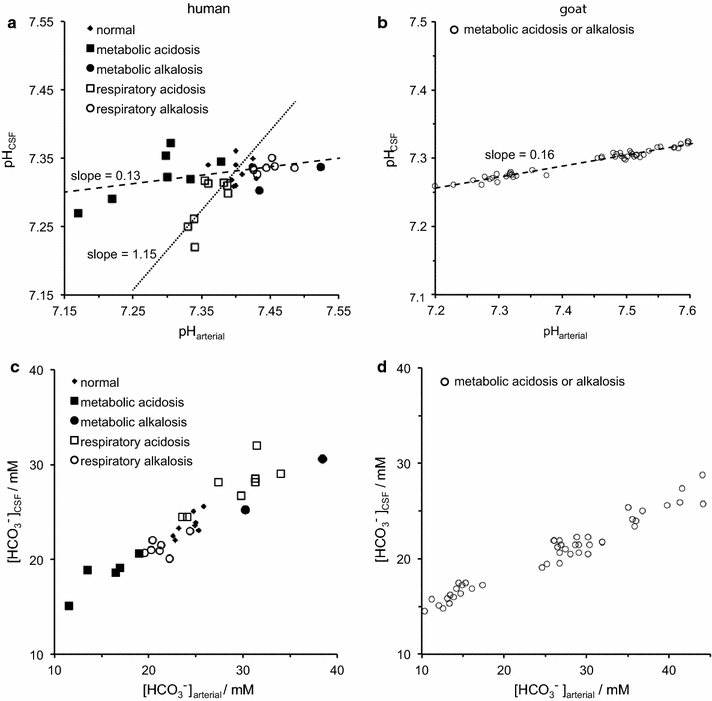
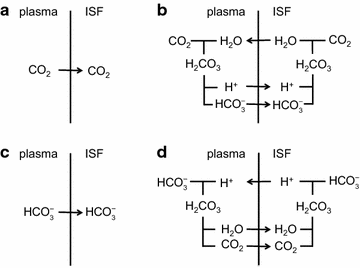
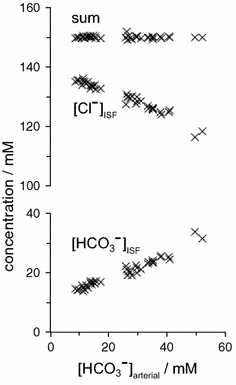
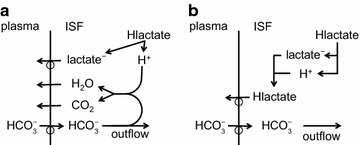
The two major interfaces separating brain and blood have different primary roles. The choroid plexuses secrete cerebrospinal fluid into the ventricles, accounting for most net fluid entry to the brain. Aquaporin, AQP1, allows water transfer across the apical surface of the choroid epithelium; another protein, perhaps GLUT1, is important on the basolateral surface. Fluid secretion is driven by apical Na+-pumps. K+ secretion occurs via net paracellular influx through relatively leaky tight junctions partially offset by transcellular efflux. The blood-brain barrier lining brain microvasculature, allows passage of O2, CO2, and glucose as required for brain cell metabolism. Because of high resistance tight junctions between microvascular endothelial cells transport of most polar solutes is greatly restricted. Because solute permeability is low, hydrostatic pressure differences cannot account for net fluid movement; however, water permeability is sufficient for fluid secretion with water following net solute transport. The endothelial cells have ion transporters that, if appropriately arranged, could support fluid secretion. Evidence favours a rate smaller than, but not much smaller than, that of the choroid plexuses. At the blood-brain barrier Na+ tracer influx into the brain substantially exceeds any possible net flux. The tracer flux may occur primarily by a paracellular route. The blood-brain barrier is the most important interface for maintaining interstitial fluid (ISF) K+ concentration within tight limits. This is most likely because Na+-pumps vary the rate at which K+ is transported out of ISF in response to small changes in K+ concentration. There is also evidence for functional regulation of K+ transporters with chronic changes in plasma concentration. The blood-brain barrier is also important in regulating HCO3- and pH in ISF: the principles of this regulation are reviewed. Whether the rate of blood-brain barrier HCO3- transport is slow or fast is discussed critically: a slow transport rate comparable to those of other ions is favoured. In metabolic acidosis and alkalosis variations in HCO3- concentration and pH are much smaller in ISF than in plasma whereas in respiratory acidosis variations in pHISF and pHplasma are similar. The key similarities and differences of the two interfaces are summarized.
Keywords: Astrocyte endfeet; Bicarbonate transport; Blood–brain barrier; Brain interstitial fluid; Cerebrospinal fluid; Chloride transport; Choroid plexus; Electroneutrality; Endothelial; Epithelial; Fluid secretion; Ion channels; Ion transporters; Neurovascular unit; Paracellular transport; Potassium regulation; Potassium transport; Sodium transport; Tight junctions; Transcellular transport; Water channels; pH regulation.
Figures


References
-
- Cserr HF. Physiology of choroid plexus. Physiol Rev. 1971;51:273–311. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

