Dosing and Pharmacokinetics of Polymyxin B in Patients with Renal Insufficiency
- PMID: 27799209
- PMCID: PMC5192162
- DOI: 10.1128/AAC.01337-16
Dosing and Pharmacokinetics of Polymyxin B in Patients with Renal Insufficiency
Abstract
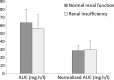
Polymyxin B remains the last-line treatment option for multidrug-resistant Gram-negative bacterial infections. Current U.S. Food and Drug Administration-approved prescribing information recommends that polymyxin B dosing should be adjusted according to the patient's renal function, despite studies that have shown poor correlation between creatinine and polymyxin B clearance. The objective of the present study was to determine whether steady-state polymyxin B exposures in patients with normal renal function were different from those in patients with renal insufficiency. Nineteen adult patients who received intravenous polymyxin B (1.5 to 2.5 mg/kg [actual body weight] daily) were included. To measure polymyxin B concentrations, serial blood samples were obtained from each patient after receiving polymyxin B for at least 48 h. The primary outcome was polymyxin B exposure at steady state, as reflected by the area under the concentration-time curve (AUC) over 24 h. Five patients had normal renal function (estimated creatinine clearance [CLCR] ≥ 80 ml/min) at baseline, whereas 14 had renal insufficiency (CLCR < 80 ml/min). The mean AUC of polymyxin B ± the standard deviation in the normal renal function cohort was 63.5 ± 16.6 mg·h/liter compared to 56.0 ± 17.5 mg·h/liter in the renal insufficiency cohort (P = 0.42). Adjusting the AUC for the daily dose (in mg/kg of actual body weight) did not result in a significant difference (28.6 ± 7.0 mg·h/liter versus 29.7 ± 11.2 mg·h/liter, P = 0.80). Polymyxin B exposures in patients with normal and impaired renal function after receiving standard dosing of polymyxin B were comparable. Polymyxin B dosing adjustment in patients with renal insufficiency should be reexamined.
Keywords: dosing adjustment; drug exposure; polymyxins.
Copyright © 2016 American Society for Microbiology.
Figures

Comment in
-
Critical Need for Clarity in Polymyxin B Dosing.Antimicrob Agents Chemother. 2017 Apr 24;61(5):e00208-17. doi: 10.1128/AAC.00208-17. Print 2017 May. Antimicrob Agents Chemother. 2017. PMID: 28438796 Free PMC article. No abstract available.
References
-
- Phe K, Lee Y, McDaneld PM, Prasad N, Yin T, Figueroa DA, Musick WL, Cottreau JM, Hu M, Tam VH. 2014. In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin B therapy. Antimicrob Agents Chemother 58:2740–2746. doi:10.1128/AAC.02476-13. - DOI - PMC - PubMed
-
- Nelson BC, Eiras DP, Gomez-Simmonds A, Loo AS, Satlin MJ, Jenkins SG, Whittier S, Calfee DP, Furuya EY, Kubin CJ. 2015. Clinical outcomes associated with polymyxin B dose in patients with bloodstream infections due to carbapenem-resistant Gram-negative rods. Antimicrob Agents Chemother 59:7000–7006. doi:10.1128/AAC.00844-15. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

