IL-27 Is Essential for Suppression of Experimental Allergic Asthma by the TLR7/8 Agonist R848 (Resiquimod)
- PMID: 27799314
- PMCID: PMC5114883
- DOI: 10.4049/jimmunol.1601094
IL-27 Is Essential for Suppression of Experimental Allergic Asthma by the TLR7/8 Agonist R848 (Resiquimod)
Abstract
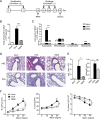
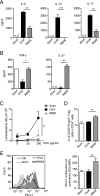
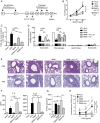
Different models of experimental allergic asthma have shown that the TLR7/8 agonist resiquimod (R848) is a potential inhibitor of type 2 helper cell-driven inflammatory responses. However, the mechanisms mediating its therapeutic effects are not fully understood. Using a model of experimental allergic asthma, we show that induction of IL-27 by R848 is critical for the observed ameliorative effects. R848 significantly inhibited all hallmarks of experimental allergic asthma, including airway hyperreactivity, eosinophilic airway inflammation, mucus hypersecretion, and Ag-specific Ig production. Whereas R848 significantly reduced IL-5, IL-13, and IL-17, it induced IFN-γ and IL-27. Neutralization of IL-27 completely reversed the therapeutic effect of R848 in the experimental asthma model, demonstrating dependence of R848-mediated suppression on IL-27. In vitro, R848 induced production of IL-27 by murine alveolar macrophages and dendritic cells and enhanced expression of programmed death-ligand 1, whose expression on monocytes and dendritic cells has been shown to regulate peripheral tolerance in both murine and human studies. Moreover, in vitro IL-27 enhanced secretion of IFN-γ whereas it inhibited IL-5 and IL-13, demonstrating its direct effect on attenuating Th2 responses. Taken together, our study proves that R848-mediated suppression of experimental asthma is dependent on IL-27. These data provide evidence of a central role of IL-27 for the control of Th2-mediated allergic diseases.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures

Similar articles
-
TLR7 Agonist Suppresses Group 2 Innate Lymphoid Cell-mediated Inflammation via IL-27-Producing Interstitial Macrophages.Am J Respir Cell Mol Biol. 2021 Sep;65(3):309-318. doi: 10.1165/rcmb.2021-0042OC. Am J Respir Cell Mol Biol. 2021. PMID: 34003734
-
The TLR7 agonist R848 alleviates allergic inflammation by targeting invariant NKT cells to produce IFN-gamma.J Immunol. 2011 Jan 1;186(1):284-90. doi: 10.4049/jimmunol.1001348. Epub 2010 Dec 3. J Immunol. 2011. PMID: 21131420
-
Treatment with the TLR7 agonist R848 induces regulatory T-cell-mediated suppression of established asthma symptoms.Eur J Immunol. 2011 Jul;41(7):1992-9. doi: 10.1002/eji.201040914. Epub 2011 Jun 6. Eur J Immunol. 2011. PMID: 21480211
-
Role of Interferon-λ in Allergic Asthma.J Innate Immun. 2015;7(3):224-30. doi: 10.1159/000369459. Epub 2015 Jan 15. J Innate Immun. 2015. PMID: 25592858 Free PMC article. Review.
-
The therapeutic potential of Toll-like receptor 7 stimulation in asthma.Inflamm Allergy Drug Targets. 2012 Dec;11(6):484-91. doi: 10.2174/187152812803589967. Inflamm Allergy Drug Targets. 2012. PMID: 23078048 Free PMC article. Review.
Cited by
-
Toll-Like Receptor Agonists as Adjuvants for Allergen Immunotherapy.Front Immunol. 2020 Nov 12;11:599083. doi: 10.3389/fimmu.2020.599083. eCollection 2020. Front Immunol. 2020. PMID: 33281825 Free PMC article. Review.
-
Costimulatory Molecules and Immune Checkpoints Are Differentially Expressed on Different Subsets of Dendritic Cells.Front Immunol. 2019 Jun 11;10:1325. doi: 10.3389/fimmu.2019.01325. eCollection 2019. Front Immunol. 2019. PMID: 31244860 Free PMC article.
-
Eleven loci with new reproducible genetic associations with allergic disease risk.J Allergy Clin Immunol. 2019 Feb;143(2):691-699. doi: 10.1016/j.jaci.2018.03.012. Epub 2018 Apr 19. J Allergy Clin Immunol. 2019. PMID: 29679657 Free PMC article.
-
Tim-3 is dispensable for allergic inflammation and respiratory tolerance in experimental asthma.PLoS One. 2021 Apr 6;16(4):e0249605. doi: 10.1371/journal.pone.0249605. eCollection 2021. PLoS One. 2021. PMID: 33822811 Free PMC article.
-
MAP3K19 Affects TWEAK-Induced Response in Cultured Bronchial Epithelial Cells and Regulates Allergic Airway Inflammation in an Asthma Murine Model.Curr Issues Mol Biol. 2023 Nov 8;45(11):8907-8924. doi: 10.3390/cimb45110559. Curr Issues Mol Biol. 2023. PMID: 37998736 Free PMC article.
References
-
- World Health Organization 2015. Chronic Respiratory Diseases. Available at: http://www.who.int/respiratory/asthma/scope/en/. Accessed: October 11, 2016.
-
- Wenzel S. E. 2012. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat. Med. 18: 716–725. - PubMed
-
- Hansel T. T., Johnston S. L., Openshaw P. J. 2013. Microbes and mucosal immune responses in asthma. Lancet 381: 861–873. - PubMed
-
- Global Initiative for Asthma 2015. Pocket Guide for Asthma Management and Prevention. Available at: http://ginasthma.org/. Accessed: October 11, 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

