The Hv1 proton channel responds to mechanical stimuli
- PMID: 27799320
- PMCID: PMC5089936
- DOI: 10.1085/jgp.201611672
The Hv1 proton channel responds to mechanical stimuli
Abstract
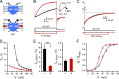
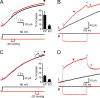
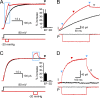
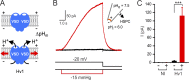
The voltage-gated proton channel, Hv1, is expressed in tissues throughout the body and plays important roles in pH homeostasis and regulation of NADPH oxidase. Hv1 operates in membrane compartments that experience strong mechanical forces under physiological or pathological conditions. In microglia, for example, Hv1 activity is potentiated by cell swelling and causes an increase in brain damage after stroke. The channel complex consists of two proton-permeable voltage-sensing domains (VSDs) linked by a cytoplasmic coiled-coil domain. Here, we report that these VSDs directly respond to mechanical stimuli. We find that membrane stretch facilitates Hv1 channel opening by increasing the rate of activation and shifting the steady-state activation curve to less depolarized potentials. In the presence of a transmembrane pH gradient, membrane stretch alone opens the channel without the need for strong depolarizations. The effect of membrane stretch persists for several minutes after the mechanical stimulus is turned off, suggesting that the channel switches to a "facilitated" mode in which opening occurs more readily and then slowly reverts to the normal mode observed in the absence of membrane stretch. Conductance simulations with a six-state model recapitulate all the features of the channel's response to mechanical stimulation. Hv1 mechanosensitivity thus provides a mechanistic link between channel activation in microglia and brain damage after stroke.
© 2016 Pathak et al.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

