pH Alkalinization by Chloroquine Suppresses Pathogenic Burkholderia Type 6 Secretion System 1 and Multinucleated Giant Cells
- PMID: 27799332
- PMCID: PMC5203652
- DOI: 10.1128/IAI.00586-16
pH Alkalinization by Chloroquine Suppresses Pathogenic Burkholderia Type 6 Secretion System 1 and Multinucleated Giant Cells
Abstract
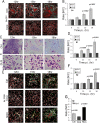
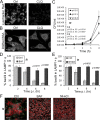
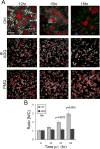
Burkholderia mallei and B. pseudomallei cause glanders and melioidosis, respectively, in humans and animals. A hallmark of pathogenesis is the formation of granulomas containing multinucleated giant cells (MNGCs) and cell death. These processes depend on type 6 secretion system 1 (T6SS-1), which is required for virulence in animals. We examined the cell biology of MNGC formation and cell death. We found that chloroquine diphosphate (CLQ), an antimalarial drug, inhibits Burkholderia growth, phagosomal escape, and subsequent MNGC formation. This depends on CLQ's ability to neutralize the acid pH because other alkalinizing compounds similarly inhibit escape and MNGC formation. CLQ inhibits bacterial virulence protein expression because T6SS-1 and some effectors of type 3 secretion system 3 (T3SS-3), which is also required for virulence, are expressed at acid pH. We show that acid pH upregulates the expression of Hcp1 of T6SS-1 and TssM, a protein coregulated with T6SS-1. Finally, we demonstrate that CLQ treatment of Burkholderia-infected Madagascar hissing cockroaches (HCs) increases their survival. This study highlights the multiple mechanisms by which CLQ inhibits growth and virulence and suggests that CLQ be further tested and considered, in conjunction with antibiotic use, for the treatment of diseases caused by Burkholderia.
Keywords: Burkholderia; Madagascar hissing cockroaches; acidification; actin tails; autophagy; chloroquine; multinucleated giant cells; phagosomal escape; type 3 secretion system; type 6 secretion system.
Copyright © 2016 American Society for Microbiology.
Figures

References
-
- Holden MT, Titball RW, Peacock SJ, Cerdeno-Tarraga AM, Atkins T, Crossman LC, Pitt T, Churcher C, Mungall K, Bentley SD, Sebaihia M, Thomson NR, Bason N, Beacham IR, Brooks K, Brown KA, Brown NF, Challis GL, Cherevach I, Chillingworth T, Cronin A, Crossett B, Davis P, DeShazer D, Feltwell T, Fraser A, Hance Z, Hauser H, Holroyd S, Jagels K, Keith KE, Maddison M, Moule S, Price C, Quail MA, Rabbinowitsch E, Rutherford K, Sanders M, Simmonds M, Songsivilai S, Stevens K, Tumapa S, Vesaratchavest M, Whitehead S, Yeats C, Barrell BG, Oyston PC, Parkhill J. 2004. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc Natl Acad Sci U S A 101:14240–14245. doi:10.1073/pnas.0403302101. - DOI - PMC - PubMed
-
- Brett PJ, DeShazer D, Woods DE. 1998. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int J Syst Bacteriol 48(Pt 1):317–320. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

