Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes
- PMID: 27799543
- PMCID: PMC5135366
- DOI: 10.1073/pnas.1613701113
Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes
Abstract
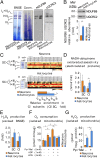
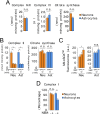
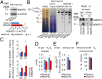
Neurons depend on oxidative phosphorylation for energy generation, whereas astrocytes do not, a distinctive feature that is essential for neurotransmission and neuronal survival. However, any link between these metabolic differences and the structural organization of the mitochondrial respiratory chain is unknown. Here, we investigated this issue and found that, in neurons, mitochondrial complex I is predominantly assembled into supercomplexes, whereas in astrocytes the abundance of free complex I is higher. The presence of free complex I in astrocytes correlates with the severalfold higher reactive oxygen species (ROS) production by astrocytes compared with neurons. Using a complexomics approach, we found that the complex I subunit NDUFS1 was more abundant in neurons than in astrocytes. Interestingly, NDUFS1 knockdown in neurons decreased the association of complex I into supercomplexes, leading to impaired oxygen consumption and increased mitochondrial ROS. Conversely, overexpression of NDUFS1 in astrocytes promoted complex I incorporation into supercomplexes, decreasing ROS. Thus, complex I assembly into supercomplexes regulates ROS production and may contribute to the bioenergetic differences between neurons and astrocytes.
Keywords: bioenergetics; brain; glycolysis; lactate; redox.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209(Pt 12):2304–2311. - PubMed
-
- Allen NJ, Barres BA. Neuroscience: Glia—more than just brain glue. Nature. 2009;457(7230):675–677. - PubMed
-
- Parpura V, et al. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369(6483):744–747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

