Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes
- PMID: 27799619
- PMCID: PMC5110024
- DOI: 10.1084/jem.20160723
Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes
Abstract
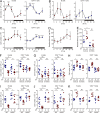
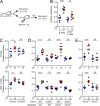
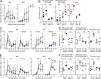
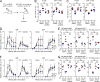
Various aspects of the immune system display circadian rhythms. Although lymphocyte trafficking has been suggested to show diurnal variations, the mechanisms and influences on immune responses are unclear. Here, we show in mice that inputs from adrenergic nerves contribute to the diurnal variation of lymphocyte recirculation through lymph nodes (LNs), which is reflected in the magnitude of the adaptive immune response. Neural inputs to β2-adrenergic receptors (β2ARs) expressed on lymphocytes reduced the frequency of lymphocyte egress from LNs at night, which was accompanied by an increase of lymphocyte numbers in LNs. Immunization during the period of lymphocyte accumulation in LNs enhanced antibody responses. The diurnal variation of the humoral immune response was dependent on β2AR-mediated neural signals and was diminished when lymphocyte recirculation through LNs was stopped. This study reveals the physiological role of adrenergic control of lymphocyte trafficking in adaptive immunity and establishes a novel mechanism that generates diurnal rhythmicity in the immune system.
© 2016 Suzuki et al.
Figures
References
-
- Curtis A.M., Fagundes C.T., Yang G., Palsson-McDermott E.M., Wochal P., McGettrick A.F., Foley N.H., Early J.O., Chen L., Zhang H., et al. . 2015. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc. Natl. Acad. Sci. USA. 112:7231–7236. 10.1073/pnas.1501327112 - DOI - PMC - PubMed
-
- Elenkov I.J., Wilder R.L., Chrousos G.P., and Vizi E.S.. 2000. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 52:595–638. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

