Physician and patient benefit-risk preferences from two randomized long-acting injectable antipsychotic trials
- PMID: 27799749
- PMCID: PMC5085312
- DOI: 10.2147/PPA.S114172
Physician and patient benefit-risk preferences from two randomized long-acting injectable antipsychotic trials
Abstract
Purpose: To quantify clinical trial participants' and investigators' judgments with respect to the relative importance of efficacy and safety attributes of antipsychotic treatments for schizophrenia, and to assess the impact of formulation and adherence.
Methods: Discrete-choice experiment surveys were completed by patients with schizophrenia and physician investigators participating in two phase-3 clinical trials of paliperidone palmitate 3-month long-acting injectable (LAI) antipsychotic. Respondents were asked to choose between hypothetical antipsychotic profiles defined by efficacy, safety, and mode of administration. Data were analyzed using random-parameters logit and probit models.
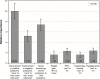
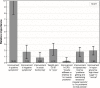
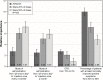
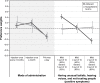
Results: Patients (N=214) and physicians (N=438) preferred complete improvement in positive symptoms (severe to none) as the most important attribute, compared with improvement in any other attribute studied. Both respondents preferred 3-month and 1-month injectables to oral formulation (P<0.05), irrespective of prior adherence to oral antipsychotic treatment, with physicians showing greater preference for a 3-month over a 1-month LAI for nonadherent patients. Physicians were willing to accept treatments with reduced efficacy for patients with prior poor adherence. The maximum decrease in efficacy (95% confidence interval [CI]) that physicians would accept for switching a patient from daily oral to 3-month injectable was as follows: adherent: 9.8% (95% CI: 7.2-12.4), 20% nonadherent: 25.4% (95% CI: 21.0-29.9), and 50% nonadherent: >30%. For patients, adherent: 10.1% (95% CI: 6.1-14.1), nonadherent: the change in efficacy studied was regarded as unimportant.
Conclusion: Improvement in positive symptoms was the most important attribute. Patients and physicians preferred LAIs over oral antipsychotics, with physicians showing a greater preference for 3-month over 1-month LAI. Physicians and patients were willing to accept reduced efficacy in exchange for switching a patient from an oral formulation to a LAI.
Keywords: benefit–risk assessment; long-acting injectable; patient preference; physician preference; schizophrenia; survey.
Conflict of interest statement
Drs Katz, Gopal, Levitan, and Weinstein are employees of Janssen and hold company stocks. Dr Levitan is also a stockholder in Baxter International, Inc., Pharmaceutical Holdrs Trust, and Zimmer Holdings, Inc. He also owns stock in a variety of companies that at times include pharmaceutical and health care–related companies. Dr Hauber is an employee of RTI Health Solutions. Drs Pugh and Fairchild were employees of RTI Health Solutions at the time this study was conducted. The authors report no other conflicts of interest in this work.
Figures
References
-
- Kane JM, Aguglia E, Altamura AC, et al. Guidelines for depot antipsychotic treatment in schizophrenia. European Neuropsychopharmacology Consensus Conference in Siena, Italy. Eur Neuropsychopharmacol. 1998;8(1):55–66. - PubMed
-
- Volavka J, Citrome L. Oral antipsychotics for the treatment of schizophrenia: heterogeneity in efficacy and tolerability should drive decision-making. Expert Opin Pharmacother. 2009;10(12):1917–1928. - PubMed
-
- Bridges JF, Mohamed AF, Finnern HW, Woehl A, Hauber AB. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer. 2012;77(1):224–231. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

