Potential of Cells and Cytokines/Chemokines to Regulate Tertiary Lymphoid Structures in Human Diseases
- PMID: 27799872
- PMCID: PMC5086451
- DOI: 10.4110/in.2016.16.5.271
Potential of Cells and Cytokines/Chemokines to Regulate Tertiary Lymphoid Structures in Human Diseases
Abstract
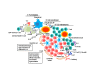
Tertiary lymphoid structures (TLS) are ectopic lymphoid tissues involved in chronic inflammation, autoimmune diseases, transplant rejection and cancer. They exhibit almost all the characteristics of secondary lymphoid organs (SLO), which are associated with adaptive immune responses; as such, they contain organized B-cell follicles with germinal centers, distinct areas containing T cells and dendritic cells, high endothelial venules, and lymphatics. In this review, we briefly describe the formation of SLO, and describe the cellular subsets and molecular cues involved in the formation and maintenance of TLS. Finally, we discuss the associations of TLS with human diseases, especially autoimmune diseases, and the potential for therapeutic targeting.
Keywords: Autoimmunity; Inflammation; Tertiary lymphoid structures.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

