Enhancement of Peripheral Nerve Regrowth by the Purine Nucleoside Analog and Cell Cycle Inhibitor, Roscovitine
- PMID: 27799897
- PMCID: PMC5066473
- DOI: 10.3389/fncel.2016.00238
Enhancement of Peripheral Nerve Regrowth by the Purine Nucleoside Analog and Cell Cycle Inhibitor, Roscovitine
Abstract
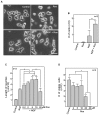
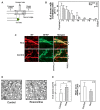
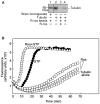
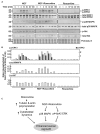
Peripheral nerve regeneration is a slow process that can be associated with limited outcomes and thus a search for novel and effective therapy for peripheral nerve injury and disease is crucial. Here, we found that roscovitine, a synthetic purine nucleoside analog, enhances neurite outgrowth in neuronal-like PC12 cells. Furthermore, ex vivo analysis of pre-injured adult rat dorsal root ganglion (DRG) neurons showed that roscovitine enhances neurite regrowth in these cells. Likewise, in vivo transected sciatic nerves in rats locally perfused with roscovitine had augmented repopulation of new myelinated axons beyond the transection zone. By mass spectrometry, we found that roscovitine interacts with tubulin and actin. It interacts directly with tubulin and causes a dose-dependent induction of tubulin polymerization as well as enhances Guanosine-5'-triphosphate (GTP)-dependent tubulin polymerization. Conversely, roscovitine interacts indirectly with actin and counteracts the inhibitory effect of cyclin-dependent kinases 5 (Cdk5) on Actin-Related Proteins 2/3 (Arp2/3)-dependent actin polymerization, and thus, causes actin polymerization. Moreover, in the presence of neurotrophic factors such as nerve growth factor (NGF), roscovitine-enhanced neurite outgrowth is mediated by increased activation of the extracellular signal-regulated kinases 1/2 (ERK1/2) and p38 mitogen-activated protein kinase (MAPK) pathways. Since microtubule and F-actin dynamics are critical for axonal regrowth, the ability of roscovitine to activate the ERK1/2 and p38 MAPK pathways and support polymerization of tubulin and actin indicate a major role for this purine nucleoside analog in the promotion of axonal regeneration. Together, our findings demonstrate a therapeutic potential for the purine nucleoside analog, roscovitine, in peripheral nerve injury.
Keywords: axon; cytoskeleton; injury; peripheral nerve; regeneration.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

