The Role of Isocitrate Lyase (ICL1) in the Metabolic Adaptation of Candida albicans Biofilms
- PMID: 27800147
- PMCID: PMC5086032
- DOI: 10.5812/jjm.38031
The Role of Isocitrate Lyase (ICL1) in the Metabolic Adaptation of Candida albicans Biofilms
Abstract
Background: A major characteristic of Candida biofilm cells that differentiates them from free-floating cells is their high tolerance to antifungal drugs. This high resistance is attributed to particular biofilm properties, including the accumulation of extrapolymeric substances, morphogenetic switching, and metabolic flexibility.
Objectives: This study evaluated the roles of metabolic processes (in particular the glyoxylate cycle) on biofilm formation, antifungal drug resistance, morphology, and cell wall components.
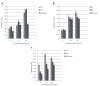
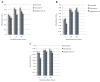
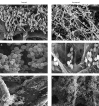
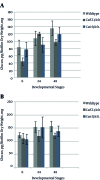
Methods: Growth, adhesion, biofilm formation, and cell wall carbohydrate composition were quantified for isogenic Candida albicans ICL1/ICL1, ICL1/icl1, and icl1/icl1 strains. The morphology and topography of these strains were compared by light microscopy and scanning electron microscopy. FKS1 (glucan synthase), ERG11 (14-α-demethylase), and CDR2 (efflux pump) mRNA levels were quantified using qRT-PCR.
Results: The ICL1/icl1 and icl1/icl1 strains formed similar biofilms and exhibited analogous drug-tolerance levels to the control ICL1/ICL1 strains. Furthermore, the drug sequestration ability of β-1, 3-glucan, a major carbohydrate component of the extracellular matrix, was not impaired. However, the inactivation of ICL1 did impair morphogenesis. ICL1 deletion also had a considerable effect on the expression of the FKS1, ERG11, and CDR2 genes. FKS1 and ERG11 were upregulated in ICL1/icl1 and icl1/icl1 cells throughout the biofilm developmental stages, and CDR2 was upregulated at the early phase. However, their expression was downregulated compared to the control ICL1/ICL1 strain.
Conclusions: We conclude that the glyoxylate cycle is not a specific determinant of biofilm drug resistance.
Keywords: Biofilms; Candida albicans; Isocitrate Lyase (ICL1); Resistance.
Figures


Similar articles
-
Resistance of Candida albicans Biofilms to Drugs and the Host Immune System.Jundishapur J Microbiol. 2016 Sep 26;9(11):e37385. doi: 10.5812/jjm.37385. eCollection 2016 Nov. Jundishapur J Microbiol. 2016. PMID: 28138373 Free PMC article. Review.
-
Transcriptional regulation of drug-resistance genes in Candida albicans biofilms in response to antifungals.J Med Microbiol. 2011 Sep;60(Pt 9):1241-1247. doi: 10.1099/jmm.0.030692-0. Epub 2011 Apr 7. J Med Microbiol. 2011. PMID: 21474609
-
Metabolic adaptation via regulated enzyme degradation in the pathogenic yeast Candida albicans.J Mycol Med. 2017 Mar;27(1):98-108. doi: 10.1016/j.mycmed.2016.12.002. Epub 2016 Dec 29. J Mycol Med. 2017. PMID: 28041812
-
Identification of Alkaloid Compounds Arborinine and Graveoline from Ruta angustifolia (L.) Pers for their Antifungal Potential against Isocitrate lyase (ICL1) gene of Candida albicans.Mycopathologia. 2021 May;186(2):221-236. doi: 10.1007/s11046-020-00523-z. Epub 2021 Feb 7. Mycopathologia. 2021. PMID: 33550536
-
Pathogenicity and drug resistance in Candida albicans and other yeast species. A review.Acta Microbiol Immunol Hung. 2007 Sep;54(3):201-35. doi: 10.1556/AMicr.54.2007.3.1. Acta Microbiol Immunol Hung. 2007. PMID: 17896473 Review.
Cited by
-
Resistance of Candida albicans Biofilms to Drugs and the Host Immune System.Jundishapur J Microbiol. 2016 Sep 26;9(11):e37385. doi: 10.5812/jjm.37385. eCollection 2016 Nov. Jundishapur J Microbiol. 2016. PMID: 28138373 Free PMC article. Review.
-
Screening and functional characterization of isocitrate lyase AceA in the biofilm formation of Vibrio alginolyticus.Appl Environ Microbiol. 2024 Nov 20;90(11):e0069724. doi: 10.1128/aem.00697-24. Epub 2024 Oct 8. Appl Environ Microbiol. 2024. PMID: 39377591 Free PMC article.
-
Glyoxylate cycle gene ICL1 is essential for the metabolic flexibility and virulence of Candida glabrata.Sci Rep. 2019 Feb 26;9(1):2843. doi: 10.1038/s41598-019-39117-1. Sci Rep. 2019. PMID: 30808979 Free PMC article.
References
-
- Calderone R, Gow NA. Host recognition by Candida species. Washington, DC: Candida and candidiasis ASM Press; 2002. pp. 67–86.
-
- Brown A, Argimon S, Gow N. Signal transduction and morphogenesis in Candida albicans. USA: Springer; 2007. pp. 167–94.
LinkOut - more resources
Full Text Sources
Other Literature Sources
