Temporal regulation of planarian eye regeneration
- PMID: 27800171
- PMCID: PMC5084360
- DOI: 10.1002/reg2.61
Temporal regulation of planarian eye regeneration
Abstract
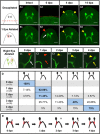
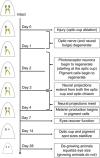
While tissue regeneration is typically studied using standard injury models, in nature injuries vary greatly in the amount and location of tissues lost. Planarians have the unique ability to regenerate from many different injuries (including from tiny fragments with no brain), allowing us to study the effects of different injuries on regeneration timelines. We followed the timing of regeneration for one organ, the eye, after multiple injury types that involved tissue loss (single- and double-eye ablation, and decapitation) in Schmidtea mediterranea. Our data reveal that the timing of regeneration remained constant despite changing injury parameters. Optic tissue regrowth, nerve re-innervation, and functional recovery were similar between injury types (even when the animal was simultaneously regrowing its brain). Changes in metabolic rate (i.e., starving vs. fed regenerates) also had no effect on regeneration timelines. In addition, our data suggest there may exist a role for optic nerve degeneration following eye ablation. Our results suggest that the temporal regulation of planarian eye regeneration is tightly controlled and resistant to variations in injury type.
Keywords: Eyes; innervation; nerve degeneration; planaria; regeneration timing.
Figures
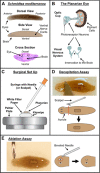
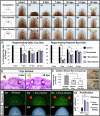
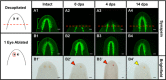

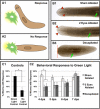

References
-
- Agata, K. , Saito, Y. & Nakajima, E. (2007). Unifying principles of regeneration I: Epimorphosis versus morphallaxis. Dev Growth Differ, 49, 73–78. - PubMed
-
- Azuma, K. & Shinozawa, T. (1998). Rhodopsin‐like proteins in planarian eye and auricle: detection and functional analysis. J Exp Biol, 201, 1263–1271. - PubMed
-
- Baguna, J. (1974). Dramatic mitotic response in planarians after feeding, and a hypothesis for the control mechanism. J Exp Zool, 190, 117–122. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
