Notch Signaling Contributes to Liver Inflammation by Regulation of Interleukin-22-Producing Cells in Hepatitis B Virus Infection
- PMID: 27800305
- PMCID: PMC5065963
- DOI: 10.3389/fcimb.2016.00132
Notch Signaling Contributes to Liver Inflammation by Regulation of Interleukin-22-Producing Cells in Hepatitis B Virus Infection
Abstract
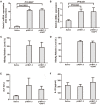
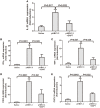
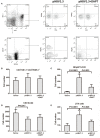
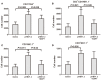
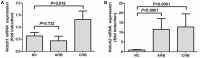
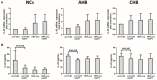
The mechanism of hepatitis B virus (HBV) induced liver inflammation is not fully elucidated. Notch signaling augmented interleukin (IL)-22 secretion in CD4+ T cells, and Notch-IL-22 axis fine-tuned inflammatory response. We previously demonstrated a proinflammatory role of IL-22 in HBV infection. Thus, in this study, we analyzed the role of Notch in development of IL-22-producing cells in HBV infection by inhibition of Notch signaling using γ-secretase inhibitor DAPT in both hydrodynamic induced HBV-infected mouse model and in peripheral blood cells isolated from patients with HBV infection. mRNA expressions of Notch1 and Notch2 were significantly increased in livers and CD4+ T cells upon HBV infection. Inhibition of Notch signaling in vivo leaded to the reduction in NKp46+ innate lymphoid cells 22 (ILC22) and lymphoid tissue inducer 4 (LTi4) cells in the liver. This process was accompanied by downregulating the expressions of IL-22 and related proinflammatory cytokines and chemokines in the liver, as well as blocking the recruitment of antigen-non-specific inflammatory cells into the liver and subsequent liver injury, but did not affect HBV antigens production and IL-22 secretion in the serum. Furthermore, IL-22 production in HBV non-specific cultured CD4+ T cells, but not HBV-specific CD4+ T cells, was reduced in response to in vitro inhibition of Notch signaling. In conclusion, Notch siganling appears to be an important mediator of the liver inflammation by modulating hepatic ILC22. The potential proinflammatory effect of Notch-mediated ILC22 may be significant for the development of new therapeutic approaches for treatment of hepatitis B.
Keywords: Notch signaling; hepatitis B virus; inflammation; innate lymphoid cells; interleukin-22.
Figures
Similar articles
-
Notch Signaling Modulates the Balance of Regulatory T Cells and T Helper 17 Cells in Patients with Chronic Hepatitis C.DNA Cell Biol. 2017 Apr;36(4):311-320. doi: 10.1089/dna.2016.3609. Epub 2017 Feb 3. DNA Cell Biol. 2017. PMID: 28157396
-
Elevated interleukin-35 suppresses liver inflammation by regulation of T helper 17 cells in acute hepatitis B virus infection.Int Immunopharmacol. 2019 May;70:252-259. doi: 10.1016/j.intimp.2019.02.048. Epub 2019 Mar 6. Int Immunopharmacol. 2019. PMID: 30851705
-
Notch Signaling Regulates Circulating T Helper 22 Cells in Patients with Chronic Hepatitis C.Viral Immunol. 2017 Sep;30(7):522-532. doi: 10.1089/vim.2017.0007. Epub 2017 Apr 14. Viral Immunol. 2017. PMID: 28410452
-
Pivotal roles of the interleukin-23/T helper 17 cell axis in hepatitis B.Liver Int. 2012 Jul;32(6):894-901. doi: 10.1111/j.1478-3231.2012.02764.x. Epub 2012 Feb 19. Liver Int. 2012. PMID: 22340646 Review.
-
[Hepatitis B virus (HBV) and the inflammatory/immune response. I. The natural environment of the antigen presentation and immunologic chaos induced by the virus].Rev Esp Enferm Dig. 1997 Dec;89(12):919-28. Rev Esp Enferm Dig. 1997. PMID: 9494379 Review. Spanish.
Cited by
-
Hepatocytes: A key role in liver inflammation.Front Immunol. 2023 Jan 18;13:1083780. doi: 10.3389/fimmu.2022.1083780. eCollection 2022. Front Immunol. 2023. PMID: 36741394 Free PMC article. Review.
-
Integration of T-cell receptor, Notch and cytokine signals programs mouse γδ T-cell effector differentiation.Immunol Cell Biol. 2018 Oct;96(9):994-1007. doi: 10.1111/imcb.12164. Epub 2018 Jun 28. Immunol Cell Biol. 2018. PMID: 29754419 Free PMC article.
-
The Role of Interleukins in HBV Infection: A Narrative Review.J Pers Med. 2023 Nov 30;13(12):1675. doi: 10.3390/jpm13121675. J Pers Med. 2023. PMID: 38138902 Free PMC article. Review.
-
The NOTCH-HES-1 axis is involved in promoting Th22 cell differentiation.Cell Mol Biol Lett. 2021 Feb 23;26(1):7. doi: 10.1186/s11658-021-00249-w. Cell Mol Biol Lett. 2021. PMID: 33622250 Free PMC article.
-
The Notch Pathway: A Link Between COVID-19 Pathophysiology and Its Cardiovascular Complications.Front Cardiovasc Med. 2021 May 26;8:681948. doi: 10.3389/fcvm.2021.681948. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34124207 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

