Erythropoietin and the use of a transgenic model of erythropoietin-deficient mice
- PMID: 27800506
- PMCID: PMC5085313
- DOI: 10.2147/HP.S83540
Erythropoietin and the use of a transgenic model of erythropoietin-deficient mice
Abstract
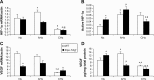
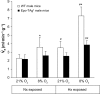
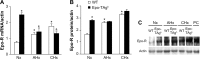
Despite its well-known role in red blood cell production, it is now accepted that erythropoietin (Epo) has other physiological functions. Epo and its receptors are expressed in many tissues, such as the brain and heart. The presence of Epo/Epo receptors in these organs suggests other roles than those usually assigned to this protein. Thus, the aim of this review is to describe the effects of Epo deficiency on adaptation to normoxic and hypoxic environments and to suggest a key role of Epo on main physiological adaptive functions. Our original model of Epo-deficient (Epo-TAgh) mice allowed us to improve our knowledge of the possible role of Epo in O2 homeostasis. The use of anemic transgenic mice revealed Epo as a crucial component of adaptation to hypoxia. Epo-TAgh mice survive well in hypoxic conditions despite low hematocrit. Furthermore, Epo plays a key role in neural control of ventilatory acclimatization and response to hypoxia, in deformability of red blood cells, in cerebral and cardiac angiogenesis, and in neuro- and cardioprotection.
Keywords: Epo-TAgh mice; hypoxia; mouse model; physiological functions.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
-
- Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2(3):336–361. - PubMed
-
- Fandrey J, Gorr TA, Gassmann M. Regulating cellular oxygen sensing by hydroxylation. Cardiovasc Res. 2006;71(4):642–651. - PubMed
-
- Yeo EJ, Chun YS, Park JW. New anticancer strategies targeting HIF-1. Biochem Pharmacol. 2004;68(6):1061–1069. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

