Erythropoietin and a nonerythropoietic peptide analog promote aortic endothelial cell repair under hypoxic conditions: role of nitric oxide
- PMID: 27800514
- PMCID: PMC5085277
- DOI: 10.2147/HP.S104377
Erythropoietin and a nonerythropoietic peptide analog promote aortic endothelial cell repair under hypoxic conditions: role of nitric oxide
Abstract
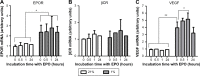
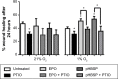
The cytoprotective effects of erythropoietin (EPO) and an EPO-related nonerythropoietic analog, pyroglutamate helix B surface peptide (pHBSP), were investigated in an in vitro model of bovine aortic endothelial cell injury under normoxic (21% O2) and hypoxic (1% O2) conditions. The potential molecular mechanisms of these effects were also explored. Using a model of endothelial injury (the scratch assay), we found that, under hypoxic conditions, EPO and pHBSP enhanced scratch closure by promoting cell migration and proliferation, but did not show any effect under normoxic conditions. Furthermore, EPO protected bovine aortic endothelial cells from staurosporine-induced apoptosis under hypoxic conditions. The priming effect of hypoxia was associated with stabilization of hypoxia inducible factor-1α, EPO receptor upregulation, and decreased Ser-1177 phosphorylation of endothelial nitric oxide synthase (NOS); the effect of hypoxia on the latter was rescued by EPO. Hypoxia was associated with a reduction in nitric oxide (NO) production as assessed by its oxidation products, nitrite and nitrate, consistent with the oxygen requirement for endogenous production of NO by endothelial NOS. However, while EPO did not affect NO formation in normoxia, it markedly increased NO production, in a manner sensitive to NOS inhibition, under hypoxic conditions. These data are consistent with the notion that the tissue-protective actions of EPO-related cytokines in pathophysiological settings associated with poor oxygenation are mediated by NO. These findings may be particularly relevant to atherogenesis and postangioplasty restenosis.
Keywords: apoptosis; erythropoietin; migration; proliferation; pyroglutamate helix B surface peptide; scratch assay.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures





Similar articles
-
Low Oxygen Tension Primes Aortic Endothelial Cells to the Reparative Effect of Tissue-Protective Cytokines.Mol Med. 2015 Dec;21(1):709-716. doi: 10.2119/molmed.2015.00162. Epub 2015 Sep 1. Mol Med. 2015. PMID: 26349058 Free PMC article.
-
Delayed administration of pyroglutamate helix B surface peptide (pHBSP), a novel nonerythropoietic analog of erythropoietin, attenuates acute kidney injury.Mol Med. 2012 May 9;18(1):719-27. doi: 10.2119/molmed.2012.00093. Mol Med. 2012. PMID: 22415011 Free PMC article.
-
Co-transactivation of the 3' erythropoietin hypoxia inducible enhancer by the HIF-1 protein.Blood Cells Mol Dis. 1997 Aug;23(2):169-76. doi: 10.1006/bcmd.1997.0134. Blood Cells Mol Dis. 1997. PMID: 9236155
-
Erythropoietin-mediated protection in kidney transplantation: nonerythropoietic EPO derivatives improve function without increasing risk of cardiovascular events.Transpl Int. 2014 Mar;27(3):241-8. doi: 10.1111/tri.12174. Epub 2013 Aug 22. Transpl Int. 2014. PMID: 23964738 Review.
-
Anemia and cerebral outcomes: many questions, fewer answers.Anesth Analg. 2008 Oct;107(4):1356-70. doi: 10.1213/ane.0b013e318184cfe9. Anesth Analg. 2008. PMID: 18806052 Review.
Cited by
-
Can Erythropoietin Reduce Hypoxemic Neurological Damages in Neonates With Congenital Heart Defects?Front Pharmacol. 2021 Nov 29;12:770590. doi: 10.3389/fphar.2021.770590. eCollection 2021. Front Pharmacol. 2021. PMID: 34912224 Free PMC article. Review.
-
Mitochondrial Metabolism as Target of the Neuroprotective Role of Erythropoietin in Parkinson's Disease.Antioxidants (Basel). 2021 Jan 15;10(1):121. doi: 10.3390/antiox10010121. Antioxidants (Basel). 2021. PMID: 33467745 Free PMC article.
-
Vascular Changes and Hypoxia in Periodontal Disease as a Link to Systemic Complications.Pathogens. 2021 Oct 5;10(10):1280. doi: 10.3390/pathogens10101280. Pathogens. 2021. PMID: 34684229 Free PMC article. Review.
-
3D Bioprinting of Novel Biocompatible Scaffolds for Endothelial Cell Repair.Polymers (Basel). 2019 Nov 22;11(12):1924. doi: 10.3390/polym11121924. Polymers (Basel). 2019. PMID: 31766610 Free PMC article.
-
PEGylated Erythropoietin Protects against Brain Injury in the MCAO-Induced Stroke Model by Blocking NF-κB Activation.Biomol Ther (Seoul). 2020 Mar 1;28(2):152-162. doi: 10.4062/biomolther.2019.147. Biomol Ther (Seoul). 2020. PMID: 31813204 Free PMC article.
References
-
- Silvestre JS, Mallat Z, Tedgui A, Levy BI. Post-ischaemic neovascularization and inflammation. Cardiovasc Res. 2008;78(2):242–249. - PubMed
-
- Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor – HIF-1 alpha in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem. 2012;19(1):90–97. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

