High-Throughput Excipient Discovery Enables Oral Delivery of Poorly Soluble Pharmaceuticals
- PMID: 27800558
- PMCID: PMC5084074
- DOI: 10.1021/acscentsci.6b00268
High-Throughput Excipient Discovery Enables Oral Delivery of Poorly Soluble Pharmaceuticals
Abstract
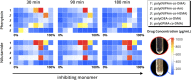
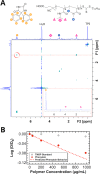
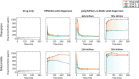
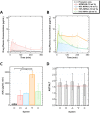
Polymeric excipients are crucial ingredients in modern pills, increasing the therapeutic bioavailability, safety, stability, and accessibility of lifesaving products to combat diseases in developed and developing countries worldwide. Because many early-pipeline drugs are clinically intractable due to hydrophobicity and crystallinity, new solubilizing excipients can reposition successful and even failed compounds to more effective and inexpensive oral formulations. With assistance from high-throughput controlled polymerization and screening tools, we employed a strategic, molecular evolution approach to systematically modulate designer excipients based on the cyclic imide chemical groups of an important (yet relatively insoluble) drug phenytoin. In these acrylamide- and methacrylate-containing polymers, a synthon approach was employed: one monomer served as a precipitation inhibitor for phenytoin recrystallization, while the comonomer provided hydrophilicity. Systems that maintained drug supersaturation in amorphous solid dispersions were identified with molecular-level understanding of noncovalent interactions using NOESY and DOSY NMR spectroscopy. Poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide) (poly(NIPAm-co-DMA)) at 70 mol % NIPAm exhibited the highest drug solubilization, in which phenytoin associated with inhibiting NIPAm units only with lowered diffusivity in solution. In vitro dissolution tests of select spray-dried dispersions corroborated the screening trends between polymer chemical composition and solubilization performance, where the best NIPAm/DMA polymer elevated the mean area-under-the-dissolution-curve by 21 times its crystalline state at 10 wt % drug loading. When administered to rats for pharmacokinetic evaluation, the same leading poly(NIPAm-co-DMA) formulation tripled the oral bioavailability compared to a leading commercial excipient, HPMCAS, and translated to a remarkable 23-fold improvement over crystalline phenytoin.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Dissolution and Solubility Enhancement of the Highly Lipophilic Drug Phenytoin via Interaction with Poly(N-isopropylacrylamide-co-vinylpyrrolidone) Excipients.Mol Pharm. 2015 Jul 6;12(7):2537-43. doi: 10.1021/acs.molpharmaceut.5b00202. Epub 2015 Jun 5. Mol Pharm. 2015. PMID: 26046484
-
Solution-state polymer assemblies influence BCS class II drug dissolution and supersaturation maintenance.Biomacromolecules. 2014 Feb 10;15(2):500-11. doi: 10.1021/bm401431t. Epub 2014 Jan 3. Biomacromolecules. 2014. PMID: 24328187
-
Deconstructing HPMCAS: Excipient Design to Tailor Polymer-Drug Interactions for Oral Drug Delivery.ACS Biomater Sci Eng. 2015 Oct 12;1(10):978-990. doi: 10.1021/acsbiomaterials.5b00234. Epub 2015 Sep 10. ACS Biomater Sci Eng. 2015. PMID: 33429529
-
Advances in Polymer Design for Enhancing Oral Drug Solubility and Delivery.Bioconjug Chem. 2018 Apr 18;29(4):939-952. doi: 10.1021/acs.bioconjchem.7b00646. Epub 2018 Jan 10. Bioconjug Chem. 2018. PMID: 29319295 Review.
-
Hydroxypropyl methylcellulose acetate succinate as an exceptional polymer for amorphous solid dispersion formulations: A review from bench to clinic.Eur J Pharm Biopharm. 2022 Aug;177:289-307. doi: 10.1016/j.ejpb.2022.07.010. Epub 2022 Jul 21. Eur J Pharm Biopharm. 2022. PMID: 35872180 Review.
Cited by
-
Amorphous Solid Dispersions: Role of the Polymer and Its Importance in Physical Stability and In Vitro Performance.Pharmaceutics. 2022 Aug 22;14(8):1747. doi: 10.3390/pharmaceutics14081747. Pharmaceutics. 2022. PMID: 36015373 Free PMC article. Review.
-
Pyrazolo-Pyrimidinones with Improved Solubility and Selective Inhibition of Adenylyl Cyclase Type 1 Activity for Treatment of Inflammatory Pain.J Med Chem. 2024 Oct 24;67(20):18290-18316. doi: 10.1021/acs.jmedchem.4c01645. Epub 2024 Oct 15. J Med Chem. 2024. PMID: 39404162
-
Random Heteropolymer Excipients Improve the Colloidal Stability of a Monoclonal Antibody for Subcutaneous Administration.Pharm Res. 2023 Feb;40(2):525-536. doi: 10.1007/s11095-022-03436-2. Epub 2022 Nov 15. Pharm Res. 2023. PMID: 36380169
-
Supersaturation and Precipitation Applicated in Drug Delivery Systems: Development Strategies and Evaluation Approaches.Molecules. 2023 Feb 27;28(5):2212. doi: 10.3390/molecules28052212. Molecules. 2023. PMID: 36903470 Free PMC article. Review.
-
A fully automated platform for photoinitiated RAFT polymerization.Digit Discov. 2023 Jan;2:219-233. doi: 10.1039/D2DD00100D. Epub 2023 Jan 5. Digit Discov. 2023. PMID: 39650094 Free PMC article.
References
-
- Sham H. L.; Kempf D. J.; Molla A.; Marsh K. C.; Kumar G. N.; Chen C. M.; Kati W.; Stewart K.; Lal R.; Hsu A.; Betebenner D.; Korneyeva M.; Vasavanonda S.; McDonald E.; Saldivar A.; Wideburg N.; Chen X.; Niu P.; Park C.; Jayanti V.; Grabowski B.; Granneman G. R.; Sun E.; Japour A. J.; Norbeck D. W. ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease. Antimicrob. Agents Chemother. 1998, 42, 3218–3224. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

