Homodimerization enhances both sensitivity and dynamic range of the ligand-binding domain of type 1 metabotropic glutamate receptor
- PMID: 27800613
- PMCID: PMC5154874
- DOI: 10.1002/1873-3468.12473
Homodimerization enhances both sensitivity and dynamic range of the ligand-binding domain of type 1 metabotropic glutamate receptor
Abstract
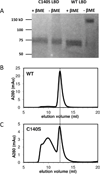
Cooperativity in ligand binding is a key emergent property of protein oligomers. Positive cooperativity (higher affinity for subsequent binding events than for initial binding) is frequent. However, the symmetrically homodimeric ligand-binding domain (LBD) of metabotropic glutamate receptor type 1 exhibits negative cooperativity. To investigate its origin and functional significance, we measured the response to glutamate in vitro of wild-type and C140S LBD as a function of the extent of dimerization. Our results indicate that homodimerization enhances the affinity of the first, but not the second, binding site, relative to the monomer, giving the dimeric receptor both greater sensitivity and a broader dynamic range.
Keywords: G-protein coupled receptor; cooperativity; ligand binding; light scattering; venus flytrap fold.
© 2016 Federation of European Biochemical Societies.
Figures



References
-
- Cornish-Bowden A. Understanding allosteric and cooperative interactions in enzymes. FEBS J. 2014;281:621–632. - PubMed
-
- Monod J, Wyman J, Changeux JP. On Nature of Allosteric Transitions - A Plausible Model. J Mol Biol. 1965;12:88&. - PubMed
-
- Koshland DE, Nemethy G, Filmer D. Comparison of Experimental Binding Data and Theoretical Models in Proteins Containing Subunits. Biochemistry. 1966;5:365-&. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

