Secondary metabolite gene clusters in the entomopathogen fungus Metarhizium anisopliae: genome identification and patterns of expression in a cuticle infection model
- PMID: 27801295
- PMCID: PMC5088523
- DOI: 10.1186/s12864-016-3067-6
Secondary metabolite gene clusters in the entomopathogen fungus Metarhizium anisopliae: genome identification and patterns of expression in a cuticle infection model
Abstract
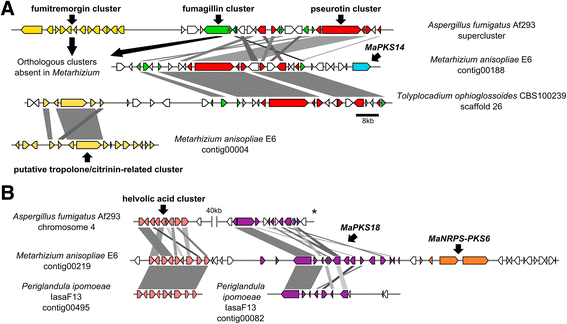
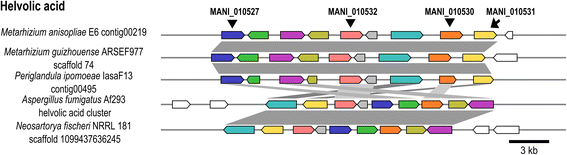
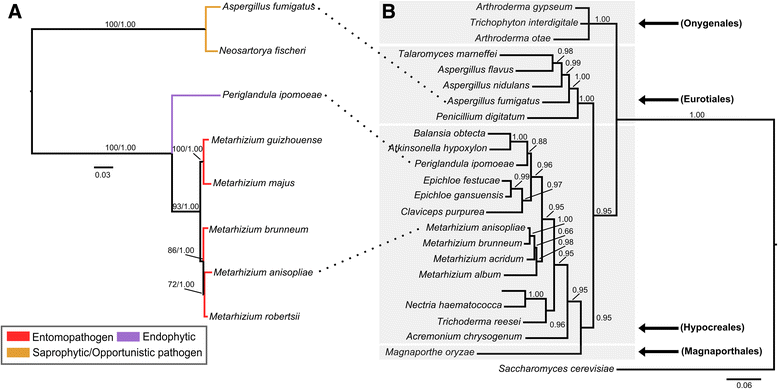
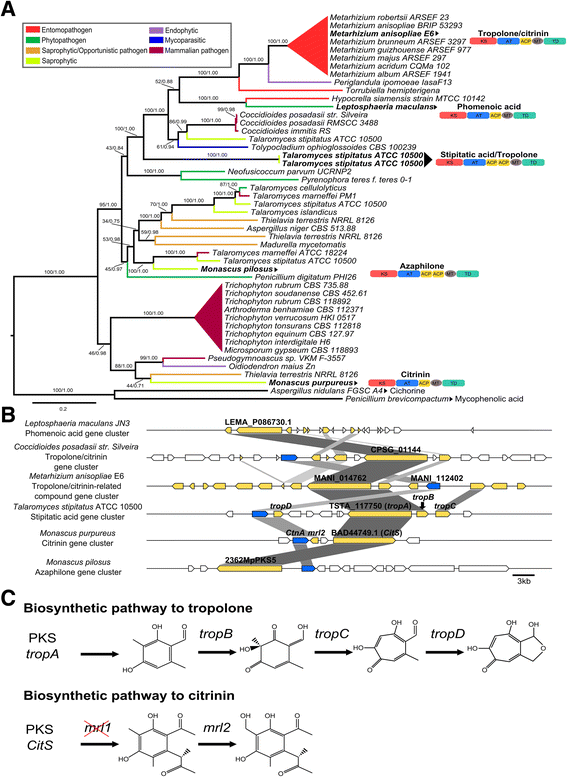
Background: The described species from the Metarhizium genus are cosmopolitan fungi that infect arthropod hosts. Interestingly, while some species infect a wide range of hosts (host-generalists), other species infect only a few arthropods (host-specialists). This singular evolutionary trait permits unique comparisons to determine how pathogens and virulence determinants emerge. Among the several virulence determinants that have been described, secondary metabolites (SMs) are suggested to play essential roles during fungal infection. Despite progress in the study of pathogen-host relationships, the majority of genes related to SM production in Metarhizium spp. are uncharacterized, and little is known about their genomic organization, expression and regulation. To better understand how infection conditions may affect SM production in Metarhizium anisopliae, we have performed a deep survey and description of SM biosynthetic gene clusters (BGCs) in M. anisopliae, analyzed RNA-seq data from fungi grown on cattle-tick cuticles, evaluated the differential expression of BGCs, and assessed conservation among the Metarhizium genus. Furthermore, our analysis extended to the construction of a phylogeny for the following three BGCs: a tropolone/citrinin-related compound (MaPKS1), a pseurotin-related compound (MaNRPS-PKS2), and a putative helvolic acid (MaTERP1).
Results: Among 73 BGCs identified in M. anisopliae, 20 % were up-regulated during initial tick cuticle infection and presumably possess virulence-related roles. These up-regulated BGCs include known clusters, such as destruxin, NG39x and ferricrocin, together with putative helvolic acid and, pseurotin and tropolone/citrinin-related compound clusters as well as uncharacterized clusters. Furthermore, several previously characterized and putative BGCs were silent or down-regulated in initial infection conditions, indicating minor participation over the course of infection. Interestingly, several up-regulated BGCs were not conserved in host-specialist species from the Metarhizium genus, indicating differences in the metabolic strategies employed by generalist and specialist species to overcome and kill their host. These differences in metabolic potential may have been partially shaped by horizontal gene transfer (HGT) events, as our phylogenetic analysis provided evidence that the putative helvolic acid cluster in Metarhizium spp. originated from an HGT event.
Conclusions: Several unknown BGCs are described, and aspects of their organization, regulation and origin are discussed, providing further support for the impact of SM on the Metarhizium genus lifestyle and infection process.
Keywords: Biological control; Cattle tick; Infection process; Metarhizium spp; Secondary metabolite biosynthetic gene clusters; Transcriptome analysis.
Figures

Similar articles
-
Genome-wide DNA methylation analysis of Metarhizium anisopliae during tick mimicked infection condition.BMC Genomics. 2019 Nov 11;20(1):836. doi: 10.1186/s12864-019-6220-1. BMC Genomics. 2019. PMID: 31711419 Free PMC article.
-
Orchestrated Biosynthesis of the Secondary Metabolite Cocktails Enables the Producing Fungus to Combat Diverse Bacteria.mBio. 2022 Oct 26;13(5):e0180022. doi: 10.1128/mbio.01800-22. Epub 2022 Aug 24. mBio. 2022. PMID: 36000736 Free PMC article.
-
Polyketides produced by the entomopathogenic fungus Metarhizium anisopliae induce Candida albicans growth.Fungal Genet Biol. 2021 Jul;152:103568. doi: 10.1016/j.fgb.2021.103568. Epub 2021 May 13. Fungal Genet Biol. 2021. PMID: 33991663
-
Molecular Genetics of Secondary Chemistry in Metarhizium Fungi.Adv Genet. 2016;94:365-436. doi: 10.1016/bs.adgen.2016.01.005. Epub 2016 Apr 7. Adv Genet. 2016. PMID: 27131330 Review.
-
Molecular interactions between entomopathogenic fungi (Hypocreales) and their insect host: Perspectives from stressful cuticle and hemolymph battlefields and the potential of dual RNA sequencing for future studies.Fungal Biol. 2018 Jun;122(6):538-545. doi: 10.1016/j.funbio.2017.10.003. Epub 2017 Oct 20. Fungal Biol. 2018. PMID: 29801798 Review.
Cited by
-
Comparative RNAseq Analysis of the Insect-Pathogenic Fungus Metarhizium anisopliae Reveals Specific Transcriptome Signatures of Filamentous and Yeast-Like Development.G3 (Bethesda). 2020 Jul 7;10(7):2141-2157. doi: 10.1534/g3.120.401040. G3 (Bethesda). 2020. PMID: 32354703 Free PMC article.
-
Genomic Analysis of the Insect-Killing Fungus Beauveria bassiana JEF-007 as a Biopesticide.Sci Rep. 2018 Aug 17;8(1):12388. doi: 10.1038/s41598-018-30856-1. Sci Rep. 2018. PMID: 30120392 Free PMC article.
-
Anticancer activity and metabolite profiling data of Penicillium janthinellum KTMT5.Data Brief. 2019 Dec 7;28:104959. doi: 10.1016/j.dib.2019.104959. eCollection 2020 Feb. Data Brief. 2019. PMID: 31890800 Free PMC article.
-
Comparative genomics reveals that metabolism underlies evolution of entomopathogenicity in bee-loving Ascosphaera spp. fungi.J Invertebr Pathol. 2022 Oct;194:107804. doi: 10.1016/j.jip.2022.107804. Epub 2022 Aug 3. J Invertebr Pathol. 2022. PMID: 35933037 Free PMC article.
-
Effect of Metarhizium anisopliae (MetA1) on growth enhancement and antioxidative defense mechanism against Rhizoctonia root rot in okra.Heliyon. 2023 Aug 12;9(8):e18978. doi: 10.1016/j.heliyon.2023.e18978. eCollection 2023 Aug. Heliyon. 2023. PMID: 37636386 Free PMC article.
References
-
- Zimmermann G. Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Sci Tech. 2007;17(9):879–920. doi: 10.1080/09583150701593963. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

