Six-month therapy for abdominal tuberculosis
- PMID: 27801499
- PMCID: PMC5450877
- DOI: 10.1002/14651858.CD012163.pub2
Six-month therapy for abdominal tuberculosis
Abstract
Background: Tuberculosis (TB) of the gastrointestinal tract and any other organ within the abdominal cavity is abdominal TB, and most guidelines recommend the same six-month regimen used for pulmonary TB for people with this diagnosis. However, some physicians are concerned whether a six-month treatment regimen is long enough to prevent relapse of the disease, particularly in people with gastrointestinal TB, which may sometimes cause antituberculous drugs to be poorly absorbed. On the other hand, longer regimens are associated with poor adherence, which could increase relapse, contribute to drug resistance developing, and increase costs to patients and health providers.
Objectives: To compare six-month versus longer drug regimens to treat people that have abdominal TB.
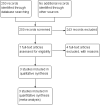
Search methods: We searched the following electronic databases up to 2 September 2016: the Cochrane Infectious Diseases Group Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Embase (accessed via OvidSP), LILACS, INDMED, and the South Asian Database of Controlled Clinical Trials. We searched the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov for ongoing trials. We also checked article reference lists.
Selection criteria: We included randomized controlled trials (RCTs) that compared six-month regimens versus longer regimens that consisted of isoniazid, rifampicin, pyrazinamide, and ethambutol to treat adults and children that had abdominal TB. The primary outcomes were relapse, with a minimum of six-month follow-up after completion of antituberculous treatment (ATT), and clinical cure at the end of ATT.
Data collection and analysis: Two review authors independently selected trials, extracted data, and assessed the risk of bias in the included trials. For analysis of dichotomous outcomes, we used risk ratios (RR) with 95% confidence intervals (CIs). Where appropriate, we pooled data from the included trials in meta-analyses. We assessed the quality of the evidence using the GRADE approach.
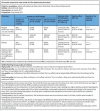
Main results: We included three RCTs, with 328 participants, that compared six-month regimens with nine-month regimens to treat adults with intestinal and peritoneal TB. All trials were conducted in Asia, and excluded people with HIV, those with co-morbidities and those who had received ATT in the previous five years. Antituberculous regimens were based on isoniazid, rifampicin, pyrazinamide, and ethambutol, and these drugs were administered daily or thrice weekly under a directly observed therapy programme. The median duration of follow-up after completion of treatment was between 12 and 39 months.Relapse was uncommon, with two cases among 140 participants treated for six months, and no events among 129 participants treated for nine months. The small number of participants means we do not know whether or not there is a difference in risk of relapse between the two regimens (very low quality evidence). At the end of therapy, there was probably no difference in the proportion of participants that achieved clinical cure between six-month and nine-month regimens (RR 1.02, 95% CI 0.97 to 1.08; 294 participants, 3 trials, moderate quality evidence). For death, there were 2/150 (1.3%) in the six-month group and 4/144 (2.8%) in the nine-month group. All deaths occurred in the first four months of treatment, so was not linked to the duration of treatment in the included trials. Similarly, the number of participants that defaulted from treatment was small in both groups, and there may be no difference between them (RR 0.50, 95% CI 0.10 to 2.59; 294 participants, 3 trials, low quality evidence). Only one trial reported on adherence to treatment, with only one participant allocated to the nine-month regimen presenting poor adherence to treatment. We do not know whether six-month regimens are associated with fewer people experiencing adverse events that lead to treatment interruption (RR 0.53, 95% CI 0.18 to 1.55; 318 participants, 3 trials, very low quality evidence).
Authors' conclusions: We found no evidence to suggest that six-month treatment regimens are inadequate for treating people that have intestinal and peritoneal TB, but numbers are small. We did not find any incremental benefits of nine-month regimens regarding relapse at the end of follow-up, or clinical cure at the end of therapy, but our confidence in the relapse estimate is very low because of size of the trials. Further research is required to make confident conclusions regarding the safety of six-month treatment for people with abdominal TB. Larger studies that include HIV-positive people, with long follow-up for detecting relapse with reliability, would help improve our knowledge around this therapeutic question.
Conflict of interest statement
SJu and HR are employed by the CIDG, which is funded by a grant from the Department for International Development (DFID), UK. SJa has no known conflicts of interest. VA is an author of one of the included trials, Makharia 2015a; he was not involved in the data extraction and the 'Risk of bias' assessment of this study.
Figures












Update of
References
References to studies included in this review
Makharia 2015a {published data only}
-
- Makharia GK, Ghoshal UC, Ramakrishna BS, Agnihotri A, Ahuja V, Chowdhury SD, et al. Intermittent directly observed therapy for abdominal tuberculosis: a multicenter randomized controlled trial comparing 6 months versus 9 months of therapy. Clinical Infectious Diseases 2015;61(5):750‐7. [DOI: 10.1093/cid/civ376] - DOI - PubMed
Park 2009 {published data only}
Tony 2008 {published data only}
-
- Tony J, Sunilkumar K, Thomas V. Randomized controlled trial of DOTS versus conventional regime for treatment of ileocecal and colonic tuberculosis. Indian Journal of Gastroenterology 2008;27(1):19‐21. - PubMed
References to studies excluded from this review
Balasubramanian 1997 {published data only}
-
- Balasubramanian R, Nagarajan M, Balambal R, Tripathy SP, Sundararaman R, Venkatesan P, et al. Randomised controlled clinical trial of short course chemotherapy in abdominal tuberculosis: a five‐year report. International Journal of Tuberculosis and Lung Disease 1997;1(1):44‐51. - PubMed
Makharia 2014a {published data only}
-
- Makharia GK, Agnihotri A, Chaudhary S, Ghoshal U, Pathak MK, Mishra A, et al. A comparison of efficacy of 6 months and 9 months of intermittent anti‐tuberculous therapy for abdominal tuberculosis (intestinal and peritoneal): a randomized controlled trial. Asian Pacific Digestive Week Conference, 2014 Nov 22‐25; Bali, Indonesia. Journal of Gastroenterology and Hepatology (Australia) 2014;29:238.
Makharia 2014b {published data only}
-
- Makharia GK, Agnihotri A, Ghoshal UC, Ramakrishna BS, Chaudhary S, Pathak MK, et al. A comparison between efficacy of 6 months and 9 months of intermittent anti‐tuberculous therapy for abdominal tuberculosis (intestinal and peritoneal): A randomized controlled trial. 55th Annual Conference of the Indian Society of Gastroenterology, ISGCON ‐ 2014, 2014 Dec 19‐23; Mumbai. Indian Journal of Gastroenterology 2014;33(1 Suppl 1):A18.
Makharia 2015b {published data only}
-
- Makharia GK, Ghoshal UC, Ramakrishna BS, Agnihotri A, Ahuja V, Chowdhury SD, et al. Intermittent supervised therapy for abdominal tuberculosis: a multicenter randomized controlled trial comparing 6 months versus 9 months duration of therapy. Version civ376v1. http://cid.oxfordjournals.org/content/early/2015/05/11/cid.civ376 (accessed 7 September 2016). [DOI: 10.1093/cid/civ376] - DOI - PubMed
Additional references
American Thoracic Society 2003
-
- American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society of America. Treatment of tuberculosis. Morbidity and Mortality Weekly Report 2003;52(RR‐11):1‐77. - PubMed
Bolukbas 2005
Debi 2014
Golden 2005
-
- Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. American Family Physician 2005;72(9):1761‐8. - PubMed
GRADEpro GDT 2014 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 06 September 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. - PubMed
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s) Cochrane Handbook for Systematic Reviews of Interventions (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
INDEX‐TB 2016
-
- INDEX‐TB Guidelines Group. INDEX‐TB Guidelines ‐ Guidelines on Extra‐Pulmonary Tuberculosis for India. http://www.icmr.nic.in/guidelines/TB/Index‐TB%20Guidelines%20‐%20green%2.... Date accessed 13th September 2016.. First. New Delhi: Central TB Division, Ministry of Health and Family Welfare, Government of India, 2016.
Jüni 2001
Khan 2006
Kim 2003
-
- Kim SG, Kim JS, Jung HC, Song IS. Is a 9‐month treatment sufficient in tuberculous enterocolitis? A prospective, randomized, single‐centre study. Alimentary Pharmacology & Therapeutics 2003;18(1):85‐91. - PubMed
Lazarus 2007
-
- Lazarus AA, Thilagar B. Abdominal tuberculosis. Disease‐a‐Month 2007;53(1):32‐8. - PubMed
Mamo 2013
-
- Mamo JP, Brij SO, Enoch DA. Abdominal tuberculosis: a retrospective review of cases presenting to a UK district hospital. Quarterly Journal of Medicine 2013;106(4):347‐54. - PubMed
Menzies 2009
Raffetseder 2014
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sharma 2004
-
- Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian Journal of Medical Research 2004;120(4):316‐53. - PubMed
Sheer 2003
-
- Sheer TA, Coyle WJ. Gastrointestinal tuberculosis. Current Gastroenterology Reports 2003;5(4):273‐8. - PubMed
Tinsa 2010
-
- Tinsa F, Essaddam L, Fitouri Z, Brini I, Douira W, Ben Becher S, et al. Abdominal tuberculosis in children. Journal of Pediatric Gastroenterology and Nutrition 2010;50(6):634‐8. - PubMed
WHO 2010
-
- World Health Organization. Guidelines for treatment of tuberculosis, 4th edition. 2010. apps.who.int/iris/bitstream/10665/44165/1/9789241547833_eng.pdf?ua=1&... (accessed 18 September 2015).
WHO 2013
-
- World Health Organization. Definitions and reporting framework for tuberculosis ‐ 2013 revision (updated December 2014). apps.who.int/iris/bitstream/10665/79199/1/9789241505345_eng.pdf (accessed 18 March 2016).
WHO 2014
-
- World Health Organization. Standards for TB care in India. 2014. www.searo.who.int/india/mediacentre/events/2014/stci_book.pdf (accessed 28 April 2016).
WHO 2015a
-
- World Health Organization. Global tuberculosis report 2015, 20th edition. apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1 (accessed 16 December 2015).
WHO 2015b
-
- World Health Organization. Use of high burden country lists for TB by WHO in the post‐2015 era. www.who.int/tb/publications/global_report/high_tb_burdencountrylists2016... (accessed 19 April 2016).
Zumla 2014
-
- Zumla AI, Gillespie SH, Hoelscher M, Philips PPJ, Cole ST, Abubakar I, et al. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet. Infectious Diseases 2014;14(4):327‐40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

