Th17 Responses to Collagen Type V, kα1-Tubulin, and Vimentin Are Present Early in Human Development and Persist Throughout Life
- PMID: 27801552
- PMCID: PMC5626015
- DOI: 10.1111/ajt.14097
Th17 Responses to Collagen Type V, kα1-Tubulin, and Vimentin Are Present Early in Human Development and Persist Throughout Life
Abstract
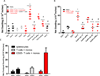
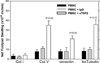
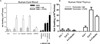
T helper 17 (Th17)-dependent autoimmune responses can develop after heart or lung transplantation and are associated with fibro-obliterative forms of chronic rejection; however, the specific self-antigens involved are typically different from those associated with autoimmune disease. To investigate the basis of these responses, we investigated whether removal of regulatory T cells or blockade of function reveals a similar autoantigen bias. We found that Th17 cells specific for collagen type V (Col V), kα1-tubulin, and vimentin were present in healthy adult peripheral blood mononuclear cells, cord blood, and fetal thymus. Using synthetic peptides and recombinant fragments of the Col V triple helical region (α1[V]), we compared Th17 cells from healthy donors with Th17 cells from Col V-reactive heart and lung patients. Although the latter responded well to α1(V) fragments and peptides in an HLA-DR-restricted fashion, Th17 cells from healthy persons responded in an HLA-DR-restricted fashion to fragments but not to peptides. Col V, kα1-tubulin, and vimentin are preferred targets of a highly conserved, hitherto unknown, preexisting Th17 response that is MHC class II restricted. These data suggest that autoimmunity after heart and lung transplantation may result from dysregulation of an intrinsic mechanism controlling airway and vascular homeostasis.
Keywords: T cell biology; basic (laboratory) research/science; cellular biology; cellular transplantation (non-islet); immunobiology; lymphocyte biology; rejection: chronic; translational research/science.
© Copyright 2016 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. David S. Wilkes is a co-founder of ImmuneWorks, Inc., a biotechnology company involved in designing therapeutics for various forms of lung diseases. The other authors have no conflicts of interest to disclose.
Figures




Similar articles
-
A shift in the collagen V antigenic epitope leads to T helper phenotype switch and immune response to self-antigen leading to chronic lung allograft rejection.Clin Exp Immunol. 2012 Jan;167(1):158-68. doi: 10.1111/j.1365-2249.2011.04486.x. Clin Exp Immunol. 2012. PMID: 22132895 Free PMC article.
-
Differential requirement for P2X7R function in IL-17 dependent vs. IL-17 independent cellular immune responses.Am J Transplant. 2014 Jul;14(7):1512-22. doi: 10.1111/ajt.12741. Epub 2014 May 27. Am J Transplant. 2014. PMID: 24866539 Free PMC article.
-
IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants.J Clin Invest. 2007 Nov;117(11):3498-506. doi: 10.1172/JCI28031. J Clin Invest. 2007. PMID: 17965778 Free PMC article.
-
Interplay between immune responses to HLA and non-HLA self-antigens in allograft rejection.Hum Immunol. 2013 Nov;74(11):1478-85. doi: 10.1016/j.humimm.2013.07.002. Epub 2013 Jul 19. Hum Immunol. 2013. PMID: 23876679 Free PMC article. Review.
-
The role of autoimmunity in obliterative bronchiolitis after lung transplantation.Am J Physiol Lung Cell Mol Physiol. 2013 Mar 1;304(5):L307-11. doi: 10.1152/ajplung.00378.2012. Epub 2012 Dec 21. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23262227 Free PMC article. Review.
Cited by
-
Infectious Tolerance as Seen With 2020 Vision: The Role of IL-35 and Extracellular Vesicles.Front Immunol. 2020 Aug 26;11:1867. doi: 10.3389/fimmu.2020.01867. eCollection 2020. Front Immunol. 2020. PMID: 32983104 Free PMC article. Review.
-
Donor HLA-DR Drives the Development of De Novo Autoimmunity Following Lung and Heart Transplantation.Transplant Direct. 2020 Sep 24;6(10):e607. doi: 10.1097/TXD.0000000000001062. eCollection 2020 Oct. Transplant Direct. 2020. PMID: 33062840 Free PMC article.
-
Metformin attenuates chronic lung allograft dysfunction: evidence in rat models.Respir Res. 2023 Jul 29;24(1):192. doi: 10.1186/s12931-023-02492-5. Respir Res. 2023. PMID: 37516880 Free PMC article.
-
Dynamic edge-based biomarker non-invasively predicts hepatocellular carcinoma with hepatitis B virus infection for individual patients based on blood testing.J Mol Cell Biol. 2019 Aug 19;11(8):665-677. doi: 10.1093/jmcb/mjz025. J Mol Cell Biol. 2019. PMID: 30925583 Free PMC article.
-
Leukocyte-Associated Ig-like Receptor 1 Inhibits Th1 Responses but Is Required for Natural and Induced Monocyte-Dependent Th17 Responses.J Immunol. 2018 Jul 15;201(2):772-781. doi: 10.4049/jimmunol.1701753. Epub 2018 Jun 8. J Immunol. 2018. PMID: 29884698 Free PMC article.
References
-
- Kotloff RM, Thabut G. Lung transplantation. Am J Respir Crit Care Med. 2011;184:159–171. - PubMed
-
- Trulock EP. Lung transplantation. Am J Respir Crit Care Med. 1997;155:789–818. - PubMed
-
- Sullivan JA, Adams AB, Burlingham WJ. The emerging role of TH17 cells in organ transplantation. Transplantation. 2014;97:483–489. - PubMed
-
- Martin JC, Baeten DL, Josien R. Emerging role of IL-17 and Th17 cells in systemic lupus erythematosus. Clin Immunol. 2014;154:1–12. - PubMed
-
- Leipe J, Grunke M, Dechant C, Reindl C, Kerzendorf U, Schulze-Koops H, Skapenko A. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876–2885. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

