A novel mechanism for host-mediated photoprotection in endosymbiotic foraminifera
- PMID: 27801906
- PMCID: PMC5270569
- DOI: 10.1038/ismej.2016.128
A novel mechanism for host-mediated photoprotection in endosymbiotic foraminifera
Abstract
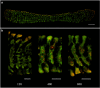
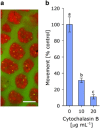
Light underpins the health and function of coral reef ecosystems, where symbiotic partnerships with photosynthetic algae constitute the life support system of the reef. Decades of research have given us detailed knowledge of the photoprotective capacity of phototrophic organisms, yet little is known about the role of the host in providing photoprotection in symbiotic systems. Here we show that the intracellular symbionts within the large photosymbiotic foraminifera Marginopora vertebralis exhibit phototactic behaviour, and that the phototactic movement of the symbionts is accomplished by the host, through rapid actin-mediated relocation of the symbionts deeper into the cavities within the calcium carbonate test. Using a photosynthetic inhibitor, we identified that the infochemical signalling for host regulation is photosynthetically derived, highlighting the presence of an intimate communication between the symbiont and the host. Our results emphasise the central importance of the host in photosymbiotic photoprotection via a new mechanism in foraminifera that can serve as a platform for exploring host-symbiont communication in other photosymbiotic organisms.
Figures



Similar articles
-
Near-future ocean acidification causes differences in microbial associations within diverse coral reef taxa.Environ Microbiol Rep. 2013 Apr;5(2):243-51. doi: 10.1111/1758-2229.12006. Epub 2012 Oct 28. Environ Microbiol Rep. 2013. PMID: 23584968
-
Evolutionary significance of the microbial assemblages of large benthic Foraminifera.Biol Rev Camb Philos Soc. 2019 Jun;94(3):828-848. doi: 10.1111/brv.12482. Epub 2018 Nov 18. Biol Rev Camb Philos Soc. 2019. PMID: 30450723 Free PMC article.
-
Patterns of species richness and the center of diversity in modern Indo-Pacific larger foraminifera.Sci Rep. 2018 May 29;8(1):8189. doi: 10.1038/s41598-018-26598-9. Sci Rep. 2018. PMID: 29844498 Free PMC article.
-
Fate of calcifying tropical symbiont-bearing large benthic foraminifera: living sands in a changing ocean.Biol Bull. 2014 Jun;226(3):169-86. doi: 10.1086/BBLv226n3p169. Biol Bull. 2014. PMID: 25070863 Review.
-
Insights into the Coral Microbiome: Underpinning the Health and Resilience of Reef Ecosystems.Annu Rev Microbiol. 2016 Sep 8;70:317-40. doi: 10.1146/annurev-micro-102215-095440. Epub 2016 Jul 8. Annu Rev Microbiol. 2016. PMID: 27482741 Review.
Cited by
-
Kleptoplast distribution, photosynthetic efficiency and sequestration mechanisms in intertidal benthic foraminifera.ISME J. 2022 Mar;16(3):822-832. doi: 10.1038/s41396-021-01128-0. Epub 2021 Oct 11. ISME J. 2022. PMID: 34635793 Free PMC article.
-
Disentangling thermal stress responses in a reef-calcifier and its photosymbionts by shotgun proteomics.Sci Rep. 2018 Feb 23;8(1):3524. doi: 10.1038/s41598-018-21875-z. Sci Rep. 2018. PMID: 29476118 Free PMC article.
-
Impact of El Niño-Southern Oscillation 2015-2016 on the soluble proteomic profile and cytolytic activity of Millepora alcicornis ("fire coral") from the Mexican Caribbean.PeerJ. 2019 Mar 18;7:e6593. doi: 10.7717/peerj.6593. eCollection 2019. PeerJ. 2019. PMID: 30918755 Free PMC article.
-
Taming the perils of photosynthesis by eukaryotes: constraints on endosymbiotic evolution in aquatic ecosystems.Commun Biol. 2023 Nov 11;6(1):1150. doi: 10.1038/s42003-023-05544-0. Commun Biol. 2023. PMID: 37952050 Free PMC article. Review.
-
Unravelling Evolutionary and Ecological Insights of Foraminifera by Using Next Generation Sequencing: A Review.Biochem Genet. 2025 Jul 15. doi: 10.1007/s10528-025-11200-5. Online ahead of print. Biochem Genet. 2025. PMID: 40665121 Review.
References
-
- Baker AC. (2003). Flexibility and specificity in coral-algal symbioses: diversity, ecology, and biogeography of Symbiodinium. Annu Rev Ecol Syst 34: 661–689.
-
- Brown BE, Ambarsari I, Warner ME, Fitt WK, Dunne RP, Gibb SW et al. (1999). Diurnal changes in photochemical efficiency and xanthophyll concentrations in shallow water reef corals: evidence for photoinhibition and photoprotection. Coral Reefs 18: 99–105.
-
- Brown BE, Downs CA, Dunne RP, Gibb SW. (2002). Preliminary evidence for tissue retraction as a factor in photoprotection of corals incapable of xanthophyll cycling. J Exp Mar Biol Ecol 277: 129–144.
-
- Carter SB. (1967). Effects of cytochalasin on mammalian cells. Nature 213: 261–264. - PubMed
-
- Dimond JL, Holzman BJ, Bingham BL. (2012). Thicker host tissues moderate light stress in a cnidarian endosymbiont. J Exp Biol 215: 2247–2254. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

