Hemoglobin-oxygen affinity in high-altitude vertebrates: is there evidence for an adaptive trend?
- PMID: 27802149
- PMCID: PMC5091379
- DOI: 10.1242/jeb.127134
Hemoglobin-oxygen affinity in high-altitude vertebrates: is there evidence for an adaptive trend?
Abstract
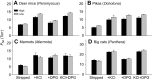
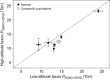
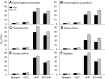
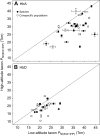
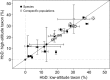
In air-breathing vertebrates at high altitude, fine-tuned adjustments in hemoglobin (Hb)-O2 affinity provide an energetically efficient means of mitigating the effects of arterial hypoxemia. However, it is not always clear whether an increased or decreased Hb-O2 affinity should be expected to improve tissue O2 delivery under different degrees of hypoxia, due to the inherent trade-off between arterial O2 loading and peripheral O2 unloading. Theoretical results indicate that the optimal Hb-O2 affinity varies as a non-linear function of environmental O2 availability, and the threshold elevation at which an increased Hb-O2 affinity becomes advantageous depends on the magnitude of diffusion limitation (the extent to which O2 equilibration at the blood-gas interface is limited by the kinetics of O2 exchange). This body of theory provides a framework for interpreting the possible adaptive significance of evolved changes in Hb-O2 affinity in vertebrates that have colonized high-altitude environments. To evaluate the evidence for an empirical generalization and to test theoretical predictions, I synthesized comparative data in a phylogenetic framework to assess the strength of the relationship between Hb-O2 affinity and native elevation in mammals and birds. Evidence for a general trend in mammals is equivocal, but there is a remarkably strong positive relationship between Hb-O2 affinity and native elevation in birds. Evolved changes in Hb function in high-altitude birds provide one of the most compelling examples of convergent biochemical adaptation in vertebrates.
Keywords: Biochemical adaptation; Blood oxygen transport; Hemoglobin; High-altitude adaptation; Hypoxia; Physiological adaptation.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The author declares no competing or financial interests.
Figures
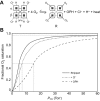

 denotes cardiac output, and
denotes cardiac output, and  denotes the rate of O2 consumption. On the right-hand side of the graph, the area of the rectangle is proportional to total O2 consumption, which can be enhanced by increasing
denotes the rate of O2 consumption. On the right-hand side of the graph, the area of the rectangle is proportional to total O2 consumption, which can be enhanced by increasing  and/or by increasing βbO2. Increases in βbO2 produce a corresponding increase in CaO2−CvO2 through shifts in the shape or position of the O2 equilibrium curve. (B) O2 equilibrium curves showing the effect of changes in Hb–O2 affinity on tissue O2 delivery under conditions of moderate hypoxia (open symbols) and severe hypoxia (filled symbols). For each pair of arterial and venous points, the PO2 for venous blood (PvO2) is marked by a vertical grey line that extends to the x-axis. The sigmoid O2 equilibrium curves are shown for high, intermediate and low Hb–O2 affinities; P50, the PO2 at which Hb is 50% saturated. Each change in Hb–O2 affinity produces a shift in PvO2, but the PO2 of arterial blood (PaO2) is assumed to remain constant. Note that under conditions of moderate hypoxia the right-shifted curve maximizes βbO2 and preserves a higher PvO2 (an overall index of tissue oxygenation). Under severe hypoxia, by contrast, the left-shifted curve maximizes βbO2 and preserves a higher PvO2 relative to the right-shifted curve. When the kinetics of O2 transfer across the alveolar gas–blood barrier is a limiting step (diffusion limitation), a left-shifted O2 equilibrium curve may also be advantageous under less severe hypoxia (Bencowitz et al., 1982).
and/or by increasing βbO2. Increases in βbO2 produce a corresponding increase in CaO2−CvO2 through shifts in the shape or position of the O2 equilibrium curve. (B) O2 equilibrium curves showing the effect of changes in Hb–O2 affinity on tissue O2 delivery under conditions of moderate hypoxia (open symbols) and severe hypoxia (filled symbols). For each pair of arterial and venous points, the PO2 for venous blood (PvO2) is marked by a vertical grey line that extends to the x-axis. The sigmoid O2 equilibrium curves are shown for high, intermediate and low Hb–O2 affinities; P50, the PO2 at which Hb is 50% saturated. Each change in Hb–O2 affinity produces a shift in PvO2, but the PO2 of arterial blood (PaO2) is assumed to remain constant. Note that under conditions of moderate hypoxia the right-shifted curve maximizes βbO2 and preserves a higher PvO2 (an overall index of tissue oxygenation). Under severe hypoxia, by contrast, the left-shifted curve maximizes βbO2 and preserves a higher PvO2 relative to the right-shifted curve. When the kinetics of O2 transfer across the alveolar gas–blood barrier is a limiting step (diffusion limitation), a left-shifted O2 equilibrium curve may also be advantageous under less severe hypoxia (Bencowitz et al., 1982).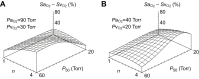
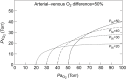
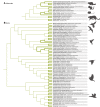
References
-
- Adair G. S. (1925). The hemoglobin system. VI. The oxygen dissociation curve of hemoglobin. J. Biol. Chem. 63, 529-545.
-
- Aste-Salazar H. and Hurtado A. (1944). The affinity of hemoglobin for oxygen at sea level and at high altitudes. Am. J. Physiol. 142, 733-743.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

