Phosphorylation of STAT2 on serine-734 negatively regulates the IFN-α-induced antiviral response
- PMID: 27802159
- PMCID: PMC6518330
- DOI: 10.1242/jcs.185421
Phosphorylation of STAT2 on serine-734 negatively regulates the IFN-α-induced antiviral response
Abstract
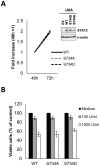
Serine phosphorylation of STAT proteins is an important post-translational modification event that, in addition to tyrosine phosphorylation, is required for strong transcriptional activity. However, we recently showed that phosphorylation of STAT2 on S287 induced by type I interferons (IFN-α and IFN-β), evoked the opposite effect. S287-STAT2 phosphorylation inhibited the biological effects of IFN-α. We now report the identification and characterization of S734 on the C-terminal transactivation domain of STAT2 as a new phosphorylation site that can be induced by type I IFNs. IFN-α-induced S734-STAT2 phosphorylation displayed different kinetics to that of tyrosine phosphorylation. S734-STAT2 phosphorylation was dependent on STAT2 tyrosine phosphorylation and JAK1 kinase activity. Mutation of S734-STAT2 to alanine (S734A) enhanced IFN-α-driven antiviral responses compared to those driven by wild-type STAT2. Furthermore, DNA microarray analysis demonstrated that a small subset of type I IFN stimulated genes (ISGs) was induced more by IFNα in cells expressing S734A-STAT2 when compared to wild-type STAT2. Taken together, these studies identify phosphorylation of S734-STAT2 as a new regulatory mechanism that negatively controls the type I IFN-antiviral response by limiting the expression of a select subset of antiviral ISGs.
Keywords: Interferon; JAK1; STAT2; Serine phosphorylation; Vesicular stomatitis; Virus infection.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures






Similar articles
-
Identification of STAT2 serine 287 as a novel regulatory phosphorylation site in type I interferon-induced cellular responses.J Biol Chem. 2013 Jan 4;288(1):747-58. doi: 10.1074/jbc.M112.402529. Epub 2012 Nov 8. J Biol Chem. 2013. PMID: 23139419 Free PMC article.
-
Chromatin dynamics of gene activation and repression in response to interferon alpha (IFN(alpha)) reveal new roles for phosphorylated and unphosphorylated forms of the transcription factor STAT2.J Biol Chem. 2011 Jun 10;286(23):20217-27. doi: 10.1074/jbc.M111.231068. Epub 2011 Apr 15. J Biol Chem. 2011. PMID: 21498520 Free PMC article.
-
Nonstructural Protein 11 of Porcine Reproductive and Respiratory Syndrome Virus Induces STAT2 Degradation To Inhibit Interferon Signaling.J Virol. 2019 Oct 29;93(22):e01352-19. doi: 10.1128/JVI.01352-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462568 Free PMC article.
-
A Positive Feedback Amplifier Circuit That Regulates Interferon (IFN)-Stimulated Gene Expression and Controls Type I and Type II IFN Responses.Front Immunol. 2018 May 28;9:1135. doi: 10.3389/fimmu.2018.01135. eCollection 2018. Front Immunol. 2018. PMID: 29892288 Free PMC article. Review.
-
The role of signal transducer and activator of transcription-2 in the interferon response.J Interferon Cytokine Res. 2012 Mar;32(3):103-10. doi: 10.1089/jir.2011.0099. Epub 2012 Jan 26. J Interferon Cytokine Res. 2012. PMID: 22280068 Free PMC article. Review.
Cited by
-
Exposing the Two Contrasting Faces of STAT2 in Inflammation.J Interferon Cytokine Res. 2022 Sep;42(9):467-481. doi: 10.1089/jir.2022.0117. Epub 2022 Jul 25. J Interferon Cytokine Res. 2022. PMID: 35877097 Free PMC article. Review.
-
Deactivation of the antiviral state by rabies virus through targeting and accumulation of persistently phosphorylated STAT1.PLoS Pathog. 2022 May 16;18(5):e1010533. doi: 10.1371/journal.ppat.1010533. eCollection 2022 May. PLoS Pathog. 2022. PMID: 35576230 Free PMC article.
-
The oncogenic mechanisms of the Janus kinase-signal transducer and activator of transcription pathway in digestive tract tumors.Cell Commun Signal. 2024 Jan 25;22(1):68. doi: 10.1186/s12964-023-01421-9. Cell Commun Signal. 2024. PMID: 38273295 Free PMC article. Review.
-
Stat2 stability regulation: an intersection between immunity and carcinogenesis.Exp Mol Med. 2020 Sep;52(9):1526-1536. doi: 10.1038/s12276-020-00506-6. Epub 2020 Sep 25. Exp Mol Med. 2020. PMID: 32973222 Free PMC article. Review.
-
Identification of a three miRNA signature as a novel potential prognostic biomarker in patients with bladder cancer.Oncotarget. 2017 Nov 6;8(62):105553-105560. doi: 10.18632/oncotarget.22318. eCollection 2017 Dec 1. Oncotarget. 2017. PMID: 29285271 Free PMC article.
References
-
- Chawla-Sarkar M., Leaman D. W. and Borden E. C. (2001). Preferential induction of apoptosis by interferon (IFN)-beta compared with IFN-alpha2: correlation with TRAIL/Apo2L induction in melanoma cell lines. Clin. Cancer Res. 7, 1821-1831. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

