Varying Degrees of Temporoparietal Hypometabolism on FDG-PET Reveal Amyloid-Positive Logopenic Primary Progressive Aphasia is not a Homogeneous Clinical Entity
- PMID: 27802232
- PMCID: PMC5894338
- DOI: 10.3233/JAD-160614
Varying Degrees of Temporoparietal Hypometabolism on FDG-PET Reveal Amyloid-Positive Logopenic Primary Progressive Aphasia is not a Homogeneous Clinical Entity
Abstract
Background: The logopenic variant of primary progressive aphasia (lvPPA) manifests due to a breakdown of the language network with prominent hypometabolism of the left temporoparietal region. LvPPA is strongly associated with amyloid deposition, yet there is question as to whether it is a homogeneous clinical entity.
Objective: This study investigated whether differences in temporoparietal metabolic patterns on 18F fludeoxyglucose positron emission tomography (FDG-PET) could elucidate brain regions preferentially affected in lvPPA.
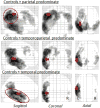
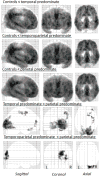
Method: We used differences in FDG-PET metabolic z-scores relative to controls for means of left lateral temporal, left inferior parietal, and left superior parietal regions to classify 53 amyloid-positive lvPPA patients into temporal, parietal, or temporoparietal predominate groups. Clinical features and FDG-PET regions of hypometabolism outside of the temporoparietal region were then compared across the three groups; the latter using statistical parametric mapping.
Results: Of the 53 lvPPA patients, 15 were classified as temporal, 14 as temporoparietal, and 22 as parietal predominate. There were no significant differences between the groups on demographic measures, language evaluation, or apolipoprotein E genotype. Compared to the other two groups, individuals with the parietal predominate pattern had extensive hypometabolism in left frontal lobe and the precuneus. Furthermore, this group had greater behavioral dyscontrol and deficits in executive function, visuospatial skills, visual memory retention, working memory, and cognitive flexibility (Bonferronip < 0.05).
Conclusions: This study demonstrates that there is clinical heterogeneity within amyloid-positive lvPPA. Patients with lvPPA with predominant parietal hypometabolism, unlike those with temporal or temporoparietal predominant hypometabolism, demonstrated widespread cognitive and behavioral changes.
Keywords: 18F fludeoxyglucose; Amyloid-β; executive function; positron emission tomography; primary progressiveaphasia; visuospatial deficit; working memory.
Conflict of interest statement
The other authors report no disclosures.
Figures

Similar articles
-
Elevated occipital β-amyloid deposition is associated with widespread cognitive impairment in logopenic progressive aphasia.J Neurol Neurosurg Psychiatry. 2013 Dec;84(12):1357-64. doi: 10.1136/jnnp-2013-305628. Epub 2013 Aug 14. J Neurol Neurosurg Psychiatry. 2013. PMID: 23946416 Free PMC article.
-
Progranulin-associated PiB-negative logopenic primary progressive aphasia.J Neurol. 2014 Mar;261(3):604-14. doi: 10.1007/s00415-014-7243-9. Epub 2014 Jan 22. J Neurol. 2014. PMID: 24449064 Free PMC article.
-
Amyloid and FDG-PET study of logopenic primary progressive aphasia: evidence for the existence of two subtypes.J Neurol. 2015 Jun;262(6):1463-72. doi: 10.1007/s00415-015-7738-z. Epub 2015 Apr 11. J Neurol. 2015. PMID: 25860346
-
Understanding the multidimensional cognitive deficits of logopenic variant primary progressive aphasia.Brain. 2022 Sep 14;145(9):2955-2966. doi: 10.1093/brain/awac208. Brain. 2022. PMID: 35857482 Free PMC article. Review.
-
FDG-PET assessment and metabolic patterns in Lafora disease.Eur J Nucl Med Mol Imaging. 2020 Jun;47(6):1576-1584. doi: 10.1007/s00259-019-04647-3. Epub 2019 Dec 19. Eur J Nucl Med Mol Imaging. 2020. PMID: 31858178
Cited by
-
Patterns of Neuropsychological Dysfunction and Cortical Volume Changes in Logopenic Aphasia.J Alzheimers Dis. 2018;66(3):1015-1025. doi: 10.3233/JAD-171175. J Alzheimers Dis. 2018. PMID: 30372673 Free PMC article.
-
Advancing Alzheimer's Therapy: Computational strategies and treatment innovations.IBRO Neurosci Rep. 2025 Feb 4;18:270-282. doi: 10.1016/j.ibneur.2025.02.002. eCollection 2025 Jun. IBRO Neurosci Rep. 2025. PMID: 39995567 Free PMC article. Review.
-
Detecting Primary Progressive Aphasia Atrophy Patterns: A Comparison of Visual Assessment and Quantitative Neuroimaging Techniques.J Alzheimers Dis Rep. 2022 Aug 5;6(1):493-501. doi: 10.3233/ADR-220036. eCollection 2022. J Alzheimers Dis Rep. 2022. PMID: 36186726 Free PMC article.
-
A Cognitive Psychometric Investigation of Word Production and Phonological Error Rates in Logopenic Progressive Aphasia.Am J Speech Lang Pathol. 2021 May 18;30(3):1194-1202. doi: 10.1044/2021_AJSLP-20-00221. Epub 2021 Apr 19. Am J Speech Lang Pathol. 2021. PMID: 33872514 Free PMC article.
-
Impaired semantic control in the logopenic variant of primary progressive aphasia.Brain Commun. 2024 Dec 21;7(1):fcae463. doi: 10.1093/braincomms/fcae463. eCollection 2025. Brain Commun. 2024. PMID: 39801715 Free PMC article.
References
-
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. - PMC - PubMed
-
- Botha H, Duffy JR, Whitwell JL, Strand EA, Machulda MM, Schwarz CG, Reid RI, Spychalla AJ, Senjem ML, Jones DT, Lowe V, Jack CR, Josephs KA. Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex; a journal devoted to the study of the nervous system and behavior. 2015;69:220–236. - PMC - PubMed
-
- Teichmann M, Kas A, Boutet C, Ferrieux S, Nogues M, Samri D, Rogan C, Dormont D, Dubois B, Migliaccio R. Deciphering logopenic primary progressive aphasia: a clinical, imaging and biomarker investigation. Brain : a journal of neurology. 2013;136:3474–3488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

