Progesterone treatment shows greater protection in brain vs. retina in a rat model of middle cerebral artery occlusion: Progesterone receptor levels may play an important role
- PMID: 27802245
- PMCID: PMC5971089
- DOI: 10.3233/RNN-160672
Progesterone treatment shows greater protection in brain vs. retina in a rat model of middle cerebral artery occlusion: Progesterone receptor levels may play an important role
Abstract
Background/objective: To determine whether inflammation increases in retina as it does in brain following middle cerebral artery occlusion (MCAO), and whether the neurosteroid progesterone, shown to have protective effects in both retina and brain after MCAO, reduces inflammation in retina as well as brain.
Methods: MCAO rats treated systemically with progesterone or vehicle were compared with shams. Protein levels of cytosolic NF-κB, nuclear NF-κB, phosphorylated NF-κB, IL-6, TNF-α, CD11b, progesterone receptor A and B, and pregnane X receptor were assessed in retinas and brains at 24 and 48 h using western blots.
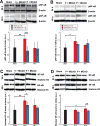
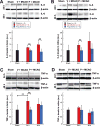
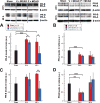
Results: Following MCAO, significant increases were observed in the following inflammatory markers: pNF-κB and CD11b at 24 h in both brain and retina, nuclear NF-κB at 24 h in brain and 48 h in retina, and TNF-α at 24 h in brain.Progesterone treatment in MCAO animals significantly attenuated levels of the following markers in brain: pNF-κB, nuclear NF-κB, IL-6, TNF-α, and CD11b, with significantly increased levels of cytosolic NF-κB. Retinas from progesterone-treated animals showed significantly reduced levels of nuclear NF-κB and IL-6 and increased levels of cytosolic NF-κB, with a trend for reduction in other markers. Post-MCAO, progesterone receptors A and B were upregulated in brain and downregulated in retina.
Conclusion: Inflammatory markers increased in both brain and retina after MCAO, with greater increases observed in brain. Progesterone treatment reduced inflammation, with more dramatic reductions observed in brain than retina. This differential effect may be due to differences in the response of progesterone receptors in brain and retina after injury.
Keywords: Focal ischemia; NF-kB; inflammation; middle cerebral artery occlusion; progesterone; progesterone receptor; rat; retina; retinal ischemia.
Figures


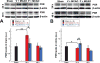
Similar articles
-
Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R.Microvasc Res. 2016 Jul;106:117-27. doi: 10.1016/j.mvr.2015.12.008. Epub 2015 Dec 12. Microvasc Res. 2016. PMID: 26686249
-
Ruscogenin reduces cerebral ischemic injury via NF-κB-mediated inflammatory pathway in the mouse model of experimental stroke.Eur J Pharmacol. 2013 Aug 15;714(1-3):303-11. doi: 10.1016/j.ejphar.2013.07.036. Epub 2013 Jul 30. Eur J Pharmacol. 2013. PMID: 23911884
-
Neuroprotective effects of pioglitazone in a rat model of permanent focal cerebral ischemia are associated with peroxisome proliferator-activated receptor gamma-mediated suppression of nuclear factor-κB signaling pathway.Neuroscience. 2011 Mar 10;176:381-95. doi: 10.1016/j.neuroscience.2010.12.029. Epub 2010 Dec 24. Neuroscience. 2011. PMID: 21185913
-
Progesterone treatment in two rat models of ocular ischemia.Invest Ophthalmol Vis Sci. 2015 May;56(5):2880-91. doi: 10.1167/iovs.14-16070. Invest Ophthalmol Vis Sci. 2015. PMID: 26024074 Free PMC article.
-
The isolated retina as a model of the CNS in pharmacology.Trends Pharmacol Sci. 1988 Mar;9(3):80-2. doi: 10.1016/0165-6147(88)90167-8. Trends Pharmacol Sci. 1988. PMID: 3072738 Review. No abstract available.
Cited by
-
Neurosteroid Receptor Modulators for Treating Traumatic Brain Injury.Neurotherapeutics. 2023 Oct;20(6):1603-1615. doi: 10.1007/s13311-023-01428-7. Epub 2023 Aug 31. Neurotherapeutics. 2023. PMID: 37653253 Free PMC article. Review.
-
Long-Term Functional and Structural Consequences of Primary Blast Overpressure to the Eye.J Neurotrauma. 2018 Sep 1;35(17):2104-2116. doi: 10.1089/neu.2017.5394. Epub 2018 Jul 2. J Neurotrauma. 2018. PMID: 29648979 Free PMC article.
-
Stem/progenitor cell-based transplantation for retinal degeneration: a review of clinical trials.Cell Death Dis. 2020 Sep 23;11(9):793. doi: 10.1038/s41419-020-02955-3. Cell Death Dis. 2020. PMID: 32968042 Free PMC article. Review.
-
The potential role of amino acids in myopia: inspiration from metabolomics.Metabolomics. 2024 Dec 15;21(1):6. doi: 10.1007/s11306-024-02207-x. Metabolomics. 2024. PMID: 39676079 Review.
-
Progesterone Attenuates Stress-Induced NLRP3 Inflammasome Activation and Enhances Autophagy following Ischemic Brain Injury.Int J Mol Sci. 2020 May 26;21(11):3740. doi: 10.3390/ijms21113740. Int J Mol Sci. 2020. PMID: 32466385 Free PMC article.
References
-
- Akiyama K, Nakanishi S, Nakamura NH, Naito T. Gene expression profiling of neuropeptides in mouse cerebellum, hippocampus, and retina. Nutrition. 2008;24(9):918–923. - PubMed
-
- Allen RS, Stein DG. Progesterone as a potential neuroprotective treatment in the retina. Expert Review of Ophthalmology. 2014;9(5):375–385. doi: 10.1586/17469899.2014.949673. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

