Acute Malaria Induces PD1+CTLA4+ Effector T Cells with Cell-Extrinsic Suppressor Function
- PMID: 27802341
- PMCID: PMC5089727
- DOI: 10.1371/journal.ppat.1005909
Acute Malaria Induces PD1+CTLA4+ Effector T Cells with Cell-Extrinsic Suppressor Function
Abstract
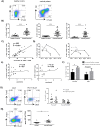
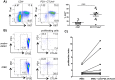
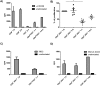
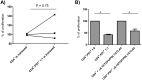
In acute Plasmodium falciparum (P. falciparum) malaria, the pro- and anti-inflammatory immune pathways must be delicately balanced so that the parasitemia is controlled without inducing immunopathology. An important mechanism to fine-tune T cell responses in the periphery is the induction of coinhibitory receptors such as CTLA4 and PD1. However, their role in acute infections such as P. falciparum malaria remains poorly understood. To test whether coinhibitory receptors modulate CD4+ T cell functions in malaria, blood samples were obtained from patients with acute P. falciparum malaria treated in Germany. Flow cytometric analysis showed a more frequent expression of CTLA4 and PD1 on CD4+ T cells of malaria patients than of healthy control subjects. In vitro stimulation with P. falciparum-infected red blood cells revealed a distinct population of PD1+CTLA4+CD4+ T cells that simultaneously produced IFNγ and IL10. This antigen-specific cytokine production was enhanced by blocking PD1/PDL1 and CTLA4. PD1+CTLA4+CD4+ T cells were further isolated based on surface expression of PD1 and their inhibitory function investigated in-vitro. Isolated PD1+CTLA4+CD4+ T cells suppressed the proliferation of the total CD4+ population in response to anti-CD3/28 and plasmodial antigens in a cell-extrinsic manner. The response to other specific antigens was not suppressed. Thus, acute P. falciparum malaria induces P. falciparum-specific PD1+CTLA4+CD4+ Teffector cells that coproduce IFNγ and IL10, and inhibit other CD4+ T cells. Transient induction of regulatory Teffector cells may be an important mechanism that controls T cell responses and might prevent severe inflammation in patients with malaria and potentially other acute infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- World Malaria Report. http://www.who.int/malaria/publications/world_malaria_report_2014/report...: World Health Organization, 2014.
-
- Mordmuller BG, Metzger WG, Juillard P, Brinkman BM, Verweij CL, Grau GE, et al. Tumor necrosis factor in Plasmodium falciparum malaria: high plasma level is associated with fever, but high production capacity is associated with rapid fever clearance. European cytokine network. 1997;8(1):29–35. Epub 1997/03/01. - PubMed
-
- Wassmer SC, Moxon CA, Taylor T, Grau GE, Molyneux ME, Craig AG. Vascular endothelial cells cultured from patients with cerebral or uncomplicated malaria exhibit differential reactivity to TNF. Cellular microbiology. 2011;13(2):198–209. Epub 2010/10/30. 10.1111/j.1462-5822.2010.01528.x - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

