Impact of SPARC expression on outcome in patients with advanced pancreatic cancer not receiving nab-paclitaxel: a pooled analysis from prospective clinical and translational trials
- PMID: 27802454
- PMCID: PMC5155356
- DOI: 10.1038/bjc.2016.355
Impact of SPARC expression on outcome in patients with advanced pancreatic cancer not receiving nab-paclitaxel: a pooled analysis from prospective clinical and translational trials
Abstract
Background: Conflicting results on the role of secreted protein acidic and rich in cysteins (SPARC) expression have been reported in resected pancreatic ductal adenocarcinoma (PDAC), and its prognostic and/or predictive role in advanced PDAC (aPDAC) has not been extensively investigated yet. This study was designed to evaluate SPARC expression as a biomarker in aPDAC patients (pts) not receiving nab-paclitaxel.
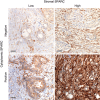
Methods: Using immunohistochemistry, we examined the stromal as well as the tumoral (i.e., cytoplasmic) SPARC expression in tumour tissue (primary tumours and metastases) of 134 aPDAC pts participating in completed prospective clinical and biomarker trials. The SPARC expression levels were correlated to the pts' clinicopathological parameters and survival times.
Results: Sixty-seven per cent of the analysed tumours showed high stromal SPARC expression, which was not associated with overall survival (OS, median 9.1 vs 7.6 months, P=0.316). A positive cytoplasmic SPARC expression was detected in 55% of the tumours and correlated significantly with inferior progression-free survival (PFS, 6.2 vs 8.6 months, P=0.004) and OS (7.8 vs 8.4 months, P=0.032). This association was strongest for pts, where primary tumour tissue was examined (PFS: 6.7 vs 10.8 months, P=0.004; OS: 7.9 vs 11.9 months, P=0.030), whereas no significant correlation was detected for pts, where only metastatic tissue was available (PFS: 5.8 vs 6.6 months, P=0.502; OS: 7.0 vs 7.8 months, P=0.452). In pts receiving gemcitabine-based chemotherapy cytoplasmic SPARC expression was significantly associated with an inferior PFS and OS (PFS: 6.2 vs 9.2 months, P=0.002; OS 7.3 vs 9.9 months, P=0.012), whereas no such association was detected for stromal SPARC expression or for pts receiving fluoropyrimidine-based chemotherapy.
Conclusion: We identified cytoplasmic SPARC expression in the primary tumour as a biomarker associated with inferior PFS and OS in aPDAC. Cytoplasmic SPARC expression may furthermore act as a negative predictive biomarker in pts treated with gemcitabine-based chemotherapy.
Figures


References
-
- Boeck S, Hoehler T, Seipelt G, Mahlberg R, Wein A, Hochhaus A, Boeck H-P, Schmid B, Kettner E, Stauch M (2008) Capecitabine plus oxaliplatin (CapOx) versus capecitabine plus gemcitabine (CapGem) versus gemcitabine plus oxaliplatin (mGemOx): final results of a multicenter randomized phase II trial in advanced pancreatic cancer. Ann Oncol 19(2): 340–347. - PubMed
-
- Boeck S, Vehling-Kaiser U, Waldschmidt D, Kettner E, Märten A, Winkelmann C, Klein S, Kojouharoff G, Gauler T, von Weikersthal LF (2010) Erlotinib 150 mg daily plus chemotherapy in advanced pancreatic cancer: an interim safety analysis of a multicenter, randomized, cross-over phase III trial of the ‘Arbeitsgemeinschaft Internistische Onkologie'. Anticancer Drugs 21(1): 94–100. - PubMed
-
- Borazanci E, Von Hoff DD (2014) Nab-paclitaxel and gemcitabine for the treatment of patients with metastatic pancreatic cancer. Expert Rev Gastroenterol Hepatol 8(7): 739–747. - PubMed
-
- Bramhall S, Schulz J, Nemunaitis J, Brown P, Baillet M, Buckels J (2002) A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer 87(2): 161–167. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

