MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 3: Brainstem involvement - frequency, presentation and outcome
- PMID: 27802825
- PMCID: PMC5088671
- DOI: 10.1186/s12974-016-0719-z
MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 3: Brainstem involvement - frequency, presentation and outcome
Abstract
Background: Myelin oligodendrocyte glycoprotein antibodies (MOG-IgG) are present in a subset of aquaporin-4 (AQP4)-IgG-negative patients with optic neuritis (ON) and/or myelitis. Little is known so far about brainstem involvement in MOG-IgG-positive patients.
Objective: To investigate the frequency, clinical and paraclinical features, course, outcome, and prognostic implications of brainstem involvement in MOG-IgG-positive ON and/or myelitis.
Methods: Retrospective case study.
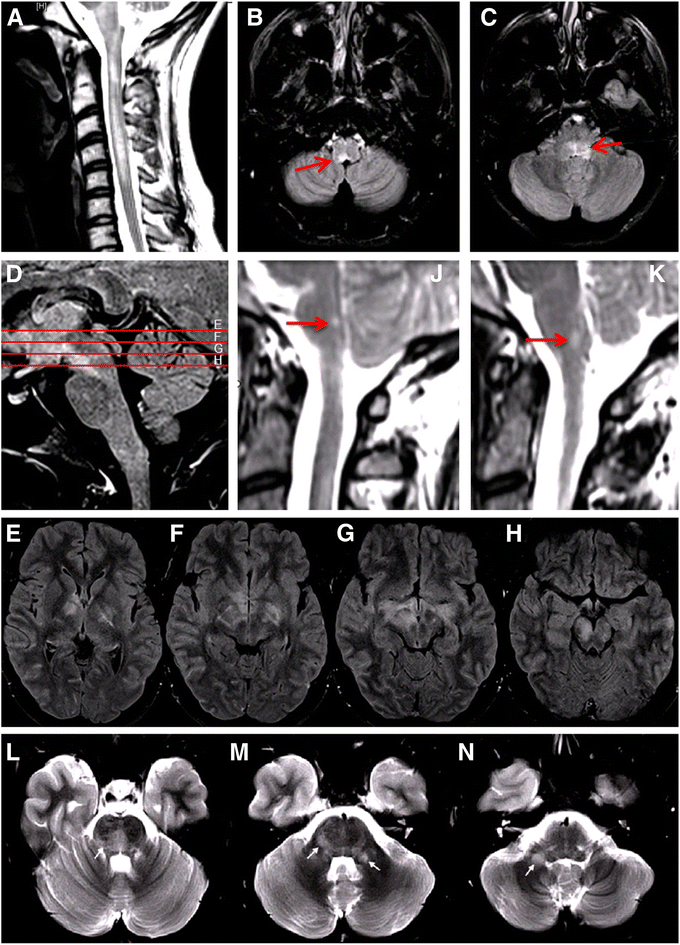
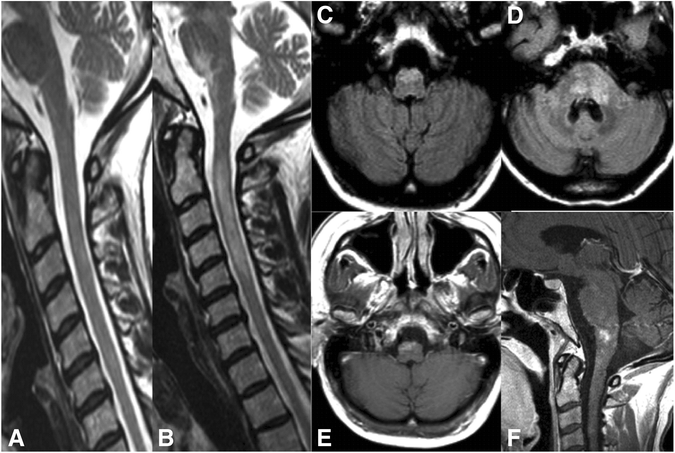
Results: Among 50 patients with MOG-IgG-positive ON and/or myelitis, 15 (30 %) with a history of brainstem encephalitis were identified. All were negative for AQP4-IgG. Symptoms included respiratory insufficiency, intractable nausea and vomiting (INV), dysarthria, dysphagia, impaired cough reflex, oculomotor nerve palsy and diplopia, nystagmus, internuclear ophthalmoplegia (INO), facial nerve paresis, trigeminal hypesthesia/dysesthesia, vertigo, hearing loss, balance difficulties, and gait and limb ataxia; brainstem involvement was asymptomatic in three cases. Brainstem inflammation was already present at or very shortly after disease onset in 7/15 (47 %) patients. 16/21 (76.2 %) brainstem attacks were accompanied by acute myelitis and/or ON. Lesions were located in the pons (11/13), medulla oblongata (8/14), mesencephalon (cerebral peduncles; 2/14), and cerebellar peduncles (5/14), were adjacent to the fourth ventricle in 2/12, and periaqueductal in 1/12; some had concomitant diencephalic (2/13) or cerebellar lesions (1/14). MRI or laboratory signs of blood-brain barrier damage were present in 5/12. Cerebrospinal fluid pleocytosis was found in 11/14 cases, with neutrophils in 7/11 (3-34 % of all CSF white blood cells), and oligoclonal bands in 4/14. Attacks were preceded by acute infection or vaccination in 5/15 (33.3 %). A history of teratoma was noted in one case. The disease followed a relapsing course in 13/15 (87 %); the brainstem was involved more than once in 6. Immunosuppression was not always effective in preventing relapses. Interferon-beta was followed by new attacks in two patients. While one patient died from central hypoventilation, partial or complete recovery was achieved in the remainder following treatment with high-dose steroids and/or plasma exchange. Brainstem involvement was associated with a more aggressive general disease course (higher relapse rate, more myelitis attacks, more frequently supratentorial brain lesions, worse EDSS at last follow-up).
Conclusions: Brainstem involvement is present in around one third of MOG-IgG-positive patients with ON and/or myelitis. Clinical manifestations are diverse and may include symptoms typically seen in AQP4-IgG-positive neuromyelitis optica, such as INV and respiratory insufficiency, or in multiple sclerosis, such as INO. As MOG-IgG-positive brainstem encephalitis may take a serious or even fatal course, particular attention should be paid to signs or symptoms of additional brainstem involvement in patients presenting with MOG-IgG-positive ON and/or myelitis.
Keywords: Aquaporin-4 antibodies (AQP4-IgG, NMO-IgG); Ataxia; Brainstem encephalitis; Cerebellitis; Diplopia Internuclear ophthalmoplegia (INO); Facial nerve palsy; Hearing loss; Intractable nausea and vomiting; Longitudinally extensive transverse myelitis (LETM); MOG-IgG; Myelin oligodendrocyte glycoprotein (MOG) antibodies; Myelitis; Neuromyelitis optica spectrum disorders (NMOSD); Optic neuritis; Respiratory insufficiency; Rhombencephalitis.
Figures
References
-
- Mader S, Gredler V, Schanda K, Rostasy K, Dujmovic I, Pfaller K, Lutterotti A, Jarius S, Di Pauli F, Kuenz B, et al. Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation. 2011;8:184. doi: 10.1186/1742-2094-8-184. - DOI - PMC - PubMed
-
- Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, Pache F, Stich O, Beume L, Hümmert MW, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflammation. 2016. doi:10.1186/s12974-016-0717-1. - PMC - PubMed
-
- Kitley J, Waters P, Woodhall M, Leite MI, Murchison A, George J, Kuker W, Chandratre S, Vincent A, Palace J. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol. 2014;71:276–83. doi: 10.1001/jamaneurol.2013.5857. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

